Figure 2.
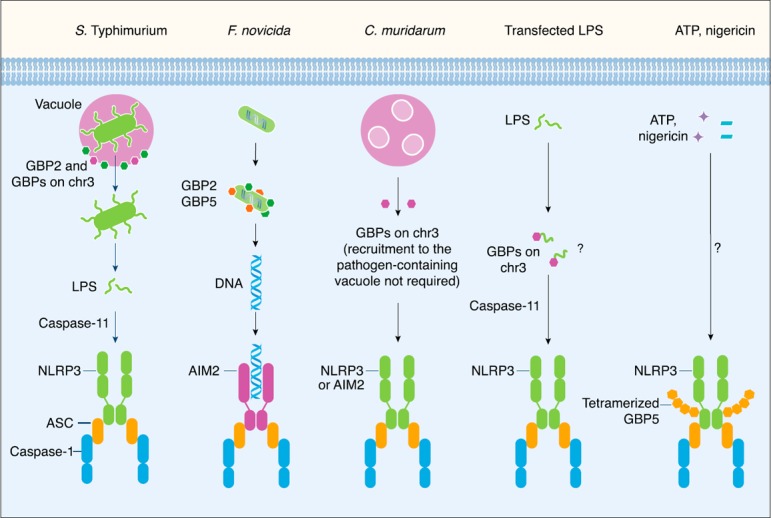
The role of GBPs in the activation of inflammasomes. The gram‐negative bacterium Salmonella Typhimurium normally resides in a vacuole. Mouse GBP2 and other GBP members within the chromosome 3 cluster (GBPs on chr3) are recruited to the vacuole where they induce vacuolar rupture. This process allows the bacteria to become cytosolic, where LPS is sensed by caspase‐11 to drive activation of the noncanonical NLRP3 inflammasome [ 37 ]. However, the gram‐negative bacterium Francisella novicida is a cytosolic pathogen that directly escapes the vacuole and does not activate caspase‐11. Mouse GBP2 and GBP5 directly target the bacteria to induce bacteriolysis and liberate bacterial DNA for sensing by the AIM2 inflammasome [ 38 , 44 ]. Mouse GBPs encoded on chromosome 3 can partially promote pyroptosis in IFN‐γ–primed BMDMs infected with the gram‐negative bacteria Chlamydia trachomatis or Chlamydia muridarum [ 42 ]. Recruitment of GBPs to the pathogen‐containing vacuole is not necessary to drive inflammasome activation in response to C. muridarum infection. There is some evidence to suggest that GBPs encoded on chromosome 3 might also induce pyroptosis in IFN‐γ‐primed BMDMs transfected with LPS [ 41 ]. However, the mechanism regulating this process remains unclear. Human and mouse GBP5 have been shown to undergo tetramerization and to interact with the pyrin domain of NLRP3. Tetramerization of GBP5 might potentiate activation of the NLRP3 inflammasome in response to the soluble NLRP3 activators ATP and nigericin, but not to crystalline NLRP3 activators [ 35 ].
