Figure 3.
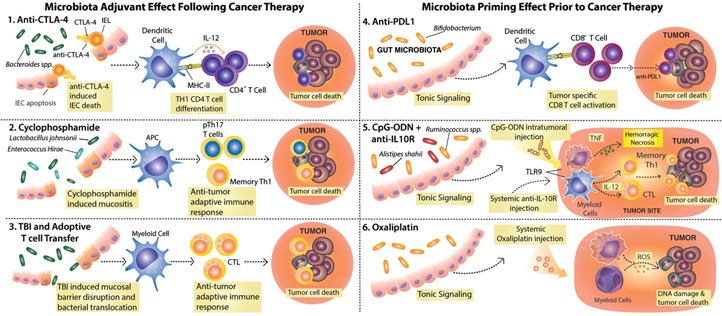
Commensal intestinal microbes modulate the response to various cancer therapies and promote spontaneous anti‐tumor immunity. (1–3) Therapies that cause damage to the intestinal epithelial barrier allow for changes in microbial composition and/or bacterial translocation and benefit from the adjuvant effect of commensal microbes. (1) Anti‐CTLA‐4 treatment leads to intraepithelial cell (IEC) apoptosis and disrupts the microbiota–host equilibrium, leading to enrichment in Bacteroides spp., which in turn, activate DCs for IL‐12 production and priming of anti‐bacteria CD4+ T cells that contribute to therapy efficacy. IEL, Intraepithelial lymphocyte; MHC‐II, MHC class II. (2) CTX induces mucositis, followed by dysbiosis and translocation of Gram‐positive bacteria that promote the induction of pTh17 and Th1 memory cells. (3) Total body irradiation (TBI), a preconditioning regime used before adoptive T cell transfer therapy, leads to mucosal barrier disruption and translocation of bacteria and bacterial products that lead to DC activation and promote the transferred cell anti‐tumor activity. (4–6) Microbiota‐derived signals prime myeloid cells for effective response to therapy. (4) Members of the Bifidobacterium genus constitutively present in the gut microbiota activate DCs, resulting in enhanced, spontaneous anti‐tumor CD8+ T cell responses. The increased frequencies of anti‐tumor CD8+ T cells in mice harboring Bifidobacterium spp. facilitate the response to anti‐PD‐L1 treatment. (5) Signals from the microbiota and in particular, Alistipes shaii and Ruminococcus spp. enable tumor‐infiltrating myeloid cells to respond to CpG‐ODN following TLR9 ligation and to produce proinflammatory cytokines (e.g., TNF, IL‐12). The CpG‐ODN activity, in combination with IL‐10R blockade (anti‐IL‐10R), leads to hemorrhagic necrosis and priming of Th1 and CTL anti‐tumor responses needed for therapy efficacy. (6) Microbiota tonic signaling conditions tumor‐infiltrating myeloid cells to produce ROS in response to oxaliplatin. ROS production by myeloid cells is required for oxaliplatin‐induced DNA damage, thus contributing to the early genotoxic effect of the drug.
