Short abstract
Intracellular trafficking of CD63 is connected to granule‐derived secretory pathways in human eosinophils.
Keywords: inflammation, immune responses, cell secretion, vesicular trafficking, transmission electron microscopy
Abstract
Eosinophil activation leads to secretion of presynthesized, granule‐stored mediators that determine the course of allergic, inflammatory, and immunoregulatory responses. CD63, a member of the transmembrane‐4 glycoprotein superfamily (tetraspanins) and present on the limiting membranes of eosinophil‐specific (secretory) granules, is considered a potential surface marker for eosinophil degranulation. However, the intracellular secretory trafficking of CD63 in eosinophils and other leukocytes is not understood. Here, we provide a comprehensive investigation of CD63 trafficking at high resolution within human eosinophils stimulated with inflammatory stimuli, CCL11 and tumor necrosis factor α, which induce distinctly differing secretory processes in eosinophils: piecemeal degranulation and compound exocytosis, respectively. By using different transmission electron microscopy approaches, including an immunonanogold technique, for enhanced detection of CD63 at subcellular compartments, we identified a major intracellular pool of CD63 that is directly linked to eosinophil degranulation events. Transmission electron microscopy quantitative analyses demonstrated that, in response to stimulation, CD63 is concentrated within granules undergoing secretion by piecemeal degranulation or compound exocytosis and that CD63 tracks with the movements of vesicles and granules in the cytoplasm. Although CD63 was observed at the cell surface after stimulation, immunonanogold electron microscopy revealed that a strong CD63 pool remains in the cytoplasm. It is remarkable that CCL11 and tumor necrosis factor α triggered increased formation of CD63+ large vesiculotubular carriers (eosinophil sombrero vesicles), which fused with granules in the process of secretion, likely acting in the intracellular translocation of CD63. Altogether, we identified active, intracellular CD63 trafficking connected to eosinophil granule‐derived secretory pathways. This is important for understanding the complex secretory activities of eosinophils underlying immune responses.
Abbreviations
- EM
= electron microscopy
- EoSV
= eosinophil sombrero vesicle
- PFA
= paraformaldehyde
- PMD
= piecemeal degranulation
- TEM
= transmission electron microscopy
Introduction
A key function of immune cells is to secrete a diversity of cytokines and other mediators that determine the course of allergic, inflammatory, and immunoregulatory responses. Thus, it is increasingly important to understand how these mediators are trafficked and secreted. The intracellular secretory compartments and the regulatory machinery that command the timing, volume, and direction of mediator release are all crucial to the coordinated delivery of these messengers (reviewed in Stow et al. [1]).
Eosinophils, leukocytes of the innate immune system, are able to release numerous mediators from their specific (secretory) granules, the major granule population in the cytoplasm of these cells. Hydrolytic enzymes; distinct, cationic proteins, including major basic protein, eosinophil peroxidase, and the eosinophil‐associated RNases: eosinophil‐derived neurotoxin and eosinophilic cationic protein; and >3 dozen cytokines with multiple functional activities are presynthesized and stored within these intracellular granules, poised for very rapid, stimulus‐induced secretion (reviewed in Spencer et al. [2]). Eosinophil‐specific granules, also termed secondary or crystalline granules, have a unique morphology, unambiguously identified by TEM and, for this reason, are both a morphologic hallmark of eosinophils and fundamental to eosinophil‐mediated responses (reviewed in Melo et al. [3]).
Structural changes of eosinophil‐specific granules are revealing in demonstrating the complex and diverse secretory activities of this cell. Fusion of a population of specific granules with each other, thus creating large, open channels for granule cargo release, characterizes a secretory process termed compound exocytosis, which is reported during the interaction of eosinophils with different parasitic helminths [4]. This pattern of secretion is also observed during the innate response by eosinophils to certain environmental fungi [5].
The identification of emptying granules with reduced electron density and disassembled contents, in the absence of granule fusions, is a feature of PMD, a secretory process frequently used by human eosinophils in a diversity of inflammatory and allergic disorders, such as asthma [6], nasal polyposis [7], allergic rhinitis [7, 8], ulcerative colitis [7], Crohn disease [7], atopic dermatitis [9], gastric carcinoma [10], shigellosis [11], and cholera [12]. In this form of secretion, human eosinophils secrete the granule matrix or the core contents or both but retain their granule containers. PMD results in a cell filled with partially empty, or fully empty, secretory granules. In contrast to compound exocytosis, whereby entire granule contents are extruded in toto, PMD enables extracellular delivery of specific mediators through transport vesicles, including large vesiculotubular carriers, termed EoSVs, which bud off from the granules to ferry their contents to the plasma membrane for release (reviewed in Melo and Weller [13]). In vitro, PMD follows stimulation with inflammatory mediators, such as the C–C chemokines CCL11 (eotaxin‐1) and CCL5 (RANTES) and platelet‐activating factor [14, 15].
CD63, a member of the transmembrane‐4 glycoprotein superfamily (tetraspanins) (reviewed in Pols and Klumperman [16]), is present on the limiting, surface membranes of eosinophil‐specific granules [15, 17, 18–19]. CD63 is also found in secretory granules of other cells from the immune system, such as human neutrophils and basophils, and constitutes a well‐established component of late endosomal and lysosomal membranes [16].
Although CD63 is associated with cell secretion and used as a surface marker for degranulation in several types of leukocytes [5, 20, 21–22], the trafficking and function of CD63 in eosinophils remain to be established. In the present work, we address the distribution and intracellular trafficking of CD63 within human eosinophils stimulated with inflammatory stimuli, which are known to induce eosinophil activation and secretion: CCL11 and TNF‐α [23, 24, 25, 26–27]. By using different TEM approaches, including an immunonanogold technique, for superior detection of CD63 at subcellular compartments and membrane microdomains [28], we provide the first characterization, to our knowledge, of intracellular, CD63‐linked secretory processes at high resolution in eosinophils from the innate immune system. We demonstrate that CD63 is concentrated on eosinophil granules actively participating in degranulation events and in transport carriers and that this tetraspanin traffics in the eosinophil cytoplasm, chaperoning both compound exocytosis and PMD.
MATERIALS AND METHODS
Eosinophil isolation, stimulation, and viability
Granulocytes were isolated from peripheral blood of allergic or healthy donors. Eosinophils were enriched and purified by negative selection with a human eosinophil‐enrichment cocktail (StemSep, StemCell Technologies, Tukwila, WA, USA) and the MACS bead procedure (Miltenyi Biotec, Auburn, CA, USA), as previously described [29], with the exception that hypotonic RBC lysis was omitted to avoid any potential for RBC lysis to affect eosinophil function. Eosinophil viability and purity were >99%, as determined by ethidium bromide (Molecular Probes, Life Technologies, Carlsbad, CA, USA) incorporation and cytocentrifuged smears stained with HEMA 3 stain kit (Thermo Fisher Scientific, Waltham, MA, USA), respectively. Experiments were approved by the Beth Israel Deaconess Medical Center, Committee on Clinical Investigation, and informed consent was obtained from all subjects. Purified eosinophils (106 cells/ml) were stimulated with TNF‐α (200 ng/ml; R&D Systems, Minneapolis, MN, USA) or recombinant human CCL11 (100 ng/ml; R&D Systems), in RPMI‐1640 medium plus 0.1% ovalbumin (Sigma‐Aldrich, St. Louis, MO, USA) or medium alone at 37°C, for 1 h. At these concentrations, CCL11 induces PMD [14] and TNF‐α induces fusion of specific granules [30].
Antibody reagents
Mouse anti‐human IgG1 CD63 (clone H5C6, catalog no. 556019) and irrelevant isotype‐control mAbs (BD Pharmingen, BD Biosciences, San Diego, CA, USA) were used for Western blotting (2 μg/ml), fluorescence microscopy (7.5 μg/ml), and electron microscopy (5 μg/ml) immunodetection studies. Rabbit anti‐human CD63 (0.25 mg/ml) (System Biosciences, Mountain View, CA, USA) or rabbit anti‐human GAPDH (0.2 mg/ml) (Sigma‐Aldrich) were additionally used for Western blotting. Secondary antibodies for Western blotting were anti‐mouse IgG HRP‐conjugated Ab (1:5000, Amersham ECL, GE Healthcare Life Sciences, Township, NJ, USA) or anti‐rabbit IgG HRP‐conjugated Ab (1:1000, Cell Signaling Technology, Danvers, MA, USA) and, for immunofluorescence, was an anti‐mouse IgG Ab conjugated to Alexa Fluor 488 (1:100; Molecular Probes). The secondary Ab for immuno‐EM was an affinity‐purified goat anti‐mouse Fab fragment conjugated to 1.4‐nm gold particles (1:100, Nanogold; Nanoprobes, Stony Brook, NY, USA).
Immunofluorescence microscopy
Human eosinophils were resuspended in 0.1% BSA RPMI‐1640 medium (1 × 106 cells/ml) and stimulated, as above, in FBS‐coated (coating: 10% FBS‐PBS [0.02 M PBS = 0.15 M NaCl], 2 h at 37°C), 96‐well plates (each, 0.2 × 106 cells/well) at 37°C in 5% CO2 incubator. Cells were removed to prewarmed Lab‐Tek II CC chamber slides (Nalge Nunc, Rochester, NY, USA), incubated for 5 min to adhere to the plate, and fixed with 3.7% PFA for 10 min at room temperature. Nonpermeabilized cells were incubated with mouse anti‐human CD63 or isotype control Ab overnight at 4°C, washed, and incubated with secondary Ab at room temperature. Cells were imaged with a BX62 Olympus upright microscope (Olympus, Tokyo, Japan), ×100 objective, coupled to a QImaging Retiga Exi‐cooled digital camera (Qimaging, Surrey, BC, Canada), and images acquired using IVision (BioVision Technologies, Exton, PA, USA).
Western blotting
Purified eosinophils (2 × 106 cells/ml) were stimulated for 1 h in the presence or absence of TNF‐α (200 ng/ml) or recombinant human CCL11 (100 ng/ml), and cell lysates (25 × 106/ml) were prepared in radioimmunoprecipitation assay buffer (Boston BioProducts, Ashland, MA, USA) with protease inhibitor cocktail (1:1000; Sigma‐Aldrich). Denatured samples were run on 4–15% Mini‐Protean TGX precast gels (Bio‐Rad Laboratories, Hercules, CA, USA), transferred to polyvinylidene difluoride membranes, and blocked for 1 h with 5% milk/TBST before probing with the primary Abs overnight at 4°C, followed by secondary Abs for 1 h at room temperature. Membranes were developed with Super Signal West Femto substrate (Thermo Fisher Scientific, Rockford, IL, USA) per the manufacturer's instructions. Signal bands were visualized using a camera and Bio‐Rad Image Lab Software.
Conventional TEM
For conventional TEM, isolated eosinophils were prepared as before [14, 15]. Cells were fixed in a mixture of freshly prepared aldehydes (1% PFA and 1.25% glutaraldehyde) in 0.1 M sodium cacodylate buffer for 1 h at room temperature, embedded in 2% agar, and kept at 4°C for further processing. Agar pellets containing eosinophils were postfixed in 1% osmium tetroxide in sym‐collidine buffer, pH 7.4, for 2 h at room temperature. After washing with sodium maleate buffer, pH 5.2, pellets were stained en bloc in 2% uranyl acetate in 0.05 M sodium maleate buffer, pH 6.0, for 2 h at room temperature and washed in the same buffer used previously before dehydration in graded ethanols and infiltration and embedding with a propylene oxide‐Epon sequence (Eponate 12 Resin; Ted Pella, Redding, CA, USA). Alternatively, samples were postfixed in 2% aqueous osmium tetroxide and 1.5% potassium ferrocyanide in 0.1 M sodium phosphate buffer, pH 6.0 (reduced osmium), before dehydration and embedding as above. After polymerization at 60°C for 16 h, thin sections were cut using a diamond knife on an ultramicrotome (Leica Microsystems, Bannockburn, IL, USA). Sections were mounted on uncoated, 200‐mesh, copper grids (Ted Pella) before staining with lead citrate and viewed with a transmission electron microscope (CM 10; Philips Research, Eindhoven, The Netherlands) at 60 KV.
Cell preparation for immunonanogold EM
For immuno‐EM, purified eosinophils were immediately fixed in fresh 4% PFA in PBS, pH 7.4 [28]. Cells were fixed for 30 min at room temperature, washed in PBS, and centrifuged at 1500 g for 1 min. Samples were then resuspended in molten 2% agar in PBS and quickly recentrifuged. Pellets were immersed in 30% sucrose in PBS overnight at 4°C, embedded in OCT compound (Miles Laboratories, Elkhart, IN, USA), and stored in −180°C liquid nitrogen for subsequent use.
Pre‐embedding immunonanogold EM
As detailed before [15, 31, 32], pre‐embedding immunolabeling was performed before standard EM processing (postfixation, dehydration, infiltration, resin embedding, and resin sectioning). All labeling steps were carried out at room temperature as before [28] as follows: 1) 1 wash in 0.02 M PBS, pH 7.6, 5 min; 2) immersion in 50 mM glycine in 0.02 M PBS, pH 7.4, 10 min; 3) incubation in a mixture of PBS and BSA (PBS‐BSA buffer; 0.02 M PBS plus 1% BSA) containing 0.1% gelatin (20 min), followed by PBS‐BSA plus 10% normal goat serum (30 min)—(this step is crucial to block nonspecific Ab binding sites); 4) incubation with primary Ab (1 h); 5) blocking with PBS‐BSA plus normal goat serum (30 min); 6) incubation with secondary Ab (1 h); 7) washing in PBS‐BSA (3 times of 5 min each); 8) postfixation in 1% glutaraldehyde (10 min); 9) 5 washings in distilled water; 10) incubation with HQ silver enhancement solution in a darkroom according to the manufacturer's instructions (Nanoprobes) (10 min) (this last step enables nucleation of the silver ions around the gold particles, and these ions precipitate as silver metal, and the particles grow in size, facilitating observation under TEM); 11) 3 washings in distilled water; 12) immersion in freshly prepared 5% sodium thiosulfate (5 min); 13) postfixation with 1% osmium tetroxide in distilled water (10 min); 14) staining with 2% uranyl acetate in distilled water (5 min); 15) embedding in Eponate (Eponate 12 Resin; Ted Pella); 16) after polymerization at 60°C for 16 h, embedding was performed by inverting eponate‐filled plastic capsules over the slide‐attached tissue sections; and 17) separation of eponate blocks from glass slides by brief immersion in liquid nitrogen. Thin sections were cut using a diamond knife on an ultramicrotome (Leica). Sections were mounted on uncoated, 200‐mesh, copper grids (Ted Pella) before staining with lead citrate and viewing with a transmission electron microscope (CM 10; Philips) at 60 kV. Two controls were performed: 1) primary Ab was replaced by an irrelevant Ab, and 2) primary Ab was omitted. Electron micrographs were randomly taken at different magnifications to study the entire cell profile and subcellular features.
Quantitative EM analysis
For quantification studies by conventional TEM (enumeration of the total number of specific granules undergoing morphologic changes of PMD or exocytosis in stimulated and unstimulated cells), we randomly took electron micrographs of cell sections showing the entire eosinophil cell profile and nucleus. A total of 87 electron micrographs (26 from unstimulated, 28 from CCL11‐stimulated, and 33 from TNF‐α–stimulated cells) and 3259 secretory granules (1090 from unstimulated, 854 from CCL11‐stimulated, and 1315 from TNF‐α–stimulated eosinophils) were counted; and the numbers of intact granules, as well as the number of granules undergoing losses of their contents indicative of PMD (with lucent areas in their cores, matrices, or both, and reduced electron density and disassembled matrices and cores), or fused granules were established as before [14].
For immunonanogold EM quantitative studies, electron micrographs randomly taken from unstimulated and stimulated eosinophils were evaluated, and the numbers of labeled and not labeled secretory granules (n = 2005 granules, 54 electron micrographs) and EoSVs (n = 1945, 23 electron micrographs) were counted in each cell section. Additionally, the numbers of labeled/unlabeled granules were correlated with the numbers of granules undergoing PMD or exocytosis. For TNF‐α–stimulated eosinophils, secretory granules (n = 460) showing pools of CD63 from 10 cells were additionally quantitated in 2 areas: peripheral cytoplasm (1.0 μm wide from the plasma membrane), corresponding to one‐third of the cell area; and within the inner cytoplasm (the contiguous cytoplasmic area deeper in the cell, corresponding to two‐thirds of the cell area). These analyses were done in clear cross‐cell sections exhibiting the entire eosinophil cell profile, intact plasma membranes, and nuclei. Lastly, 175 secretory granules showing pools of CD63 from CCL11‐stimulated or TNF‐α–stimulated cells and controls (n = 29 cells) were analyzed for quantification of the total granule area and area occupied by the CD63 immunolabeling in each granule.
All quantitative studies were performed using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
One‐way or 2‐way ANOVA followed by Tukey multiple comparisons test or the Student's t test was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA, USA). Additionally, the normal distribution analysis (Shapiro‐Wilk test) was used to evaluate the total area of the secretory granules and the area occupied by CD63. Significance was P < 0.05.
RESULTS
Extracellular labeling of CD63 reveals different patterns of immunoreactivity
In previous works, we and others demonstrated by immunofluorescence that CD63 is localized on the periphery of the resting major basic protein–positive secretory granules from human eosinophils in both intact, permeabilized cells and isolated by subcellular fractionation [14, 15, 17]. While examining extracellular labeling of CD63 in intact nonpermeabilized human eosinophils, stimulated or not with degranulation stimuli for 1 h (CCL11 or TNF‐α), we clearly noticed different patterns of immunoreactivity for CD63 at the cell surface ( Fig. 1A–C ). Unstimulated cells showed weak or no fluorescence (Fig. 1A). CCL11 induced punctate, bright labeling (Fig. 1B), with focal immunofluorescent spots resembling secretion through PMD [24, 25], whereas a mostly diffuse fluorescence was observed in response to TNF‐α stimulation (Fig. 1C). Controls in which the primary Ab was replaced by an irrelevant antibody were negative (not shown).
Figure 1.

CD63 Immunolabeling in nonpermeabilized human eosinophils. Although unstimulated cells (A) show absent or weak fluorescence, a pool of CD63 imaged as green fluorescence is observed at the surface of cells stimulated with CCL11 (B) or TNF‐α (C). Note that the immunoreactivity pattern is punctate with CCL11 (B) and mostly diffuse with TNF‐α (C). Eosinophils were isolated by negative selection from the blood of healthy human donors, stimulated with CCL11 (B) or TNF‐α (C) for 1 h, and incubated with mouse anti‐human CD63 or isotype antibody, followed by secondary antibody (anti‐mouse conjugated with Alexa Fluor 488). Control cells were kept in medium (A). Images are representative of 3 independent experiments. Scale bar, 2.3 μm (A–C).
Because both CCL11 and TNF‐α are robust stimuli that induce eosinophil secretion [27, 31, 33, 34], the presence of CD63 at the cell surface indicates that 1) this tetraspanin was mobilized from intracellular pools to the external cell surface in response to stimulation, and 2) the surface expression of CD63, imaged through distinct patterns of fluorescence, might be associated with different secretory events.
CCL11 and TNF‐α induce distinct secretory processes in human eosinophils
TEM is the only technique with resolution sufficient to clearly identify and distinguish between different modes of cell secretion [13]. To characterize ultrastructural events within secretory granules that underlie agonist‐elicited secretion, freshly isolated human eosinophils were stimulated with CCL11 or TNF‐α or kept in medium alone for 1 h, immediately fixed while still in suspension, and prepared for conventional TEM. As expected, cells stimulated with CCL11 showed a morphologic pattern of PMD, characterized by cytoplasmic vesiculation and progressive emptying of the contents from granule cores, surrounding matrices, or both, in the absence of granule fusions ( Fig. 2A ), as previously demonstrated [14]. Moreover, emptying granules with morphologic features of PMD were always intermingled, in the same cell section, with resting, nonmobilized granules (Fig. 2A), a hallmark of PMD [13, 14]. In contrast, TNF‐α triggered a different secretory process—compound exocytosis—characterized by fusion of a number of granules with each other, leading to formation of large channels in the cytoplasm (Fig. 2B–D). These fused granules had clear losses of their contents (Fig. 2B–D). Connectivity between fused granules and the plasma membrane was observed and only the first granule of the channel was generally fused with the plasma membrane (Fig. 2D). The occurrence of TNF‐α‐induced compound exocytosis was also confirmed in samples prepared for TEM with a step of postfixation with reduced osmium, which increases granule membrane contrast, thus highlighting granule‐fusion events (Supplemental Fig. 1).
Figure 2.

Conventional TEM identifies distinct, secretory processes, triggered by CCL11 and TNF‐α stimulation. (A) PMD, characterized by progressive emptying of the secretory granules in the absence of granule fusions, was observed in response to CCL11. In (Ai–Aiii), note, in high magnification, the disarrangement of the granule cores and matrices. EoSVs, highlighted in pink, are seen around emptying granules. (B and C) Compound exocytosis, characterized by large channels formed by granule–granule fusions, was the predominant mode of secretion induced by TNF‐α. In (D), granule losses and fusion of the first granule from the channel with the plasma membrane (arrow) are shown. (E) Significant increases in numbers of emptying or fused granules occurred after stimulation with CCL11 or TNF‐α, respectively. Eosinophils were isolated from the peripheral blood by negative selection, immediately fixed, and processed for conventional TEM. Counts were derived from 3 experiments with 3259 granules counted in 87 electron micrographs randomly taken and showing the entire cell profile and nucleus. Scale bar, 700 nm (A), 315 nm (Ai–Aiii), 860 nm (B), 1.1 μm (C), and 580 nm (D). Data represent means ± sem ****P < 0.0001 vs. control intact granules; #### P < 0.0001 vs. control‐emptying granules; ++++ P < 0.0001 vs. control‐fused granules.
To quantify the number of granules undergoing PMD or compound exocytosis, eosinophil sections showing the entire cell profile and nucleus were evaluated (n = 87), and a total of 3259 granules were analyzed. Eosinophil activation induced significant increases in the numbers of granules exhibiting morphologic changes. PMD was the predominant event found in CCL11‐stimulated cells (13.0 ± 1.7 granules/cell section, corresponding to 47.9 ± 6.1% of the total number of granules, means ± sem, n = 28 cells), whereas TNF‐α induced mostly compound exocytosis (26.7 ± 1.9 granules/cell section, corresponding to 65.5 ± 1.9%, means ± sem, n = 33 cells) (Fig. 2E). PMD was also found, to a lesser degree, in cells stimulated with TNF‐α (Fig. 2E). In unstimulated cells, most granules were intact (38.1 ± 1.8 granules/cell section, corresponding to 90.6 ± 1.3% of the total number of granules, means ± sem, n = 26 cells) (Fig. 2E).
CD63 is strongly associated with PMD and compound exocytosis
To address the granule‐associated distribution and trafficking of CD63 within CCL11‐stimulated or TNF‐α–stimulated eosinophils, we next performed ultrastructural labeling of this tetraspanin with a pre‐embedding immunonanogold EM technique for optimal antigen preservation [28]. First, the numbers of CD63‐labeled and not labeled secretory granules were quantitated. In all groups, most specific granules were positive for CD63 ( Fig. 3A ).
Figure 3.

CD63 immunolabeling of secretory granules by immunonanogold EM. (A) Quantitative EM analyses revealed that most secretory granules were positive for CD63 in all groups. Stimulation led to significant increase in the numbers of CD63‐labeled granules compared to unstimulated cells. (B) A representative electron micrograph of an unstimulated human eosinophil revealed CD63 labeling on the granules (Gr) limiting membranes. The boxed area in (B) is shown in higher magnification in (Bi). A CD63+ EoSV is indicated (arrow). Eosinophils were isolated from the peripheral blood, stimulated or not with CCL11 or TNF‐α and prepared for pre‐embedding immunanogold EM. Counts were derived from, at least, 3 experiments with 2005 granules counted in 54 electron micrographs randomly taken and showing the entire cell profile and nucleus (N). Scale bars, 800 nm (B); 500 nm (Bi). ****P < 0.0001 vs. control cells.
In unstimulated cells, CD63 was localized primarily on the cytoplasmic surface of the secretory granules’ limiting membranes, as demonstrated before by our group [15] (Fig. 3B and Bi). EoSVs were also labeled for CD63 (Fig. 3Bi, arrow).
CCL11 led to strong CD63 labeling of granules undergoing loss of their contents through PMD ( Fig. 4A ). These granules had different degrees of emptying and were uniformly distributed in the cytoplasm (Fig. 4A). CCL11 induced accumulation of CD63 within emptying granules, and intragranular CD63 pools were clearly seen (Fig. 4A and Supplementary Fig. 2). Labeling for CD63 was also observed at transport vesicles around, in contact with, or even inside, secretory granules, including EoSVs (Supplemental Fig. 2). A CD63+ “tail,” budding from secretory granules, likely a budding tubular vesicle, was frequently observed in thin sections of eosinophils (Supplemental Fig. 2). The presence of intact granules negative for CD63 close to highly labeled granules (Fig. 4A and Supplemental Fig. 2) and CD63 labeling at the cell surface (Supplemental Fig. 3) were noted.
Figure 4.
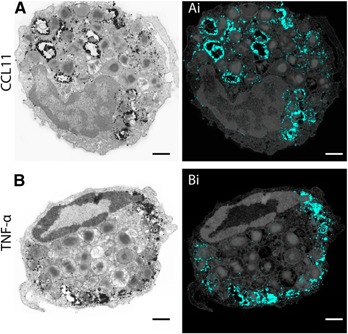
CD63 is strongly associated with secretory processes within human eosinophils. (A, B) Secretory granules (Gr) undergoing content release through PMD (A) or compound exocytosis (B) were intensely labeled for CD63. Note that although CD63+ granules were distributed in the entire cytoplasm in PMD (A), these organelles were concentrated in the peripheral cytoplasm in compound exocytosis (B). In (Ai and Bi), granules were highlighted in blue using Photoshop software. Cells were isolated from the peripheral blood, stimulated with CCL11 (A) or TNF‐α (B) and prepared for pre‐embedding immunanogold EM. Scale bars, 915 nm (A, Ai), 680 nm (B, Bi).
Stimulation of eosinophils with TNF‐α induced an immunolabeling pattern for CD63 markedly different than for CCL11. TNF‐α–triggered compound exocytosis led to a robust labeling of granules mostly confined at the cell periphery, near the plasma membrane, whereas granules localized in the inner cytoplasm were not, or were weakly, labeled for CD63 (Fig. 4B). This immunolabeling pattern was very consistent, with different cell sections in the same field showing exactly the same aspect ( Fig. 5 ). Large clusters of CD63 immunoreactivity were associated with channels or enlarged chambers formed by granule–granule fusions (Figs. 4 and 5). These channels/chambers had several residual cores or reduced electron‐density, or both, indicative of content losses (Fig. 5). Interestingly, on granules localized beneath the plasma membrane, we frequently noticed a polarization of the CD63 immunolabeling at the granule side facing the plasma membrane (Fig. 5, arrowheads).
Figure 5.

Differential distribution of CD63+ secretory granules within human eosinophils after stimulation with TNF‐α. (A) A representative electron micrograph shows several cell sections with cytoplasmic CD63+ granules, mostly confined at the cell periphery, near the plasma membrane. Arrowheads indicate CD63 polarization on the granule face toward the plasma membrane. (B) Quantitative analyses of immunolabeled granules. Eosinophils were isolated from the peripheral blood, stimulated with TNF‐α, and prepared for pre‐embedding immunonanogold EM. A total of 460 granules from 10 sharp, cross‐cell sections exhibiting the entire cell profile, intact plasma membrane, and nucleus were counted in the peripheral cytoplasm (1.0 μm wide from the plasma membrane, as indicated by the red bars) and within the inner cytoplasm (the contiguous cytoplasmic area deeper in the cell). Scale bar, 1.3 μm. ****P < 0.0001 vs. CD63+ granules at cell inner area; #### P < 0.0001 vs. CD63− granules at cell inner area.
Next, because we noticed a marked difference in the cytoplasmic distribution of CD63+ granules in TNF‐α–stimulated cells, we performed quantitative studies to develop more insight into CD63 intracellular trafficking. Granules showing pools of CD63 were counted in the peripheral cytoplasm of eosinophil cross sections, within a 1.0‐μm‐wide “belt” from the plasma membrane, corresponding to one‐third of the cell area, as showed in Fig. 5A (red bars), and within the inner cytoplasm (the contiguous cytoplasmic area deeper in the cell, corresponding to two‐thirds of the cell area). Quantitative EM revealed that 75.0 ± 4.5% (means ± sem, n = 460 granules) of the granules with pools of CD63 were localized at the cell periphery, whereas just 16.2 ± 5.2% (means ± sem, n = 460 granules) of these organelles were distributed in the inner cytoplasm (Fig. 5B). Most secretory granules in the inner cytoplasm (83.7 ± 5.2%) showed an absence, or very weak labeling, for CD63 (Fig. 5B). These quantitative data reinforce that CD63 traffics in the cytoplasm in concert with the movement of granules involved in compound exocytosis.
Control cells, from all conditions, in which the primary Ab was omitted or replaced by an irrelevant Ab were negative (Supplemental Fig. 4).
CD63 concentrates within granules actively participating in degranulation events
We next evaluated whether the eosinophil cellular content of CD63 changed in stimulated, compared with unstimulated, eosinophils. Western blotting analyses showed high levels of CD63 within eosinophils in all conditions that were similar in unstimulated and stimulated cells ( Fig. 6A ). This result was also observed with the use of an additional primary anti‐CD63 Ab (data not shown).
Figure 6.

CD63 concentrates within granules undergoing active processes of secretion. (A) Representative CD63 expression in non‐stimulated (NS), CCL11‐ or TNF‐α‐stimulated human eosinophils evaluated by western blotting (n = 3). L = lane. (B) Representative images of secretory granules at high resolution within human eosinophils after stimulation or not. Note that CD63 was concentrated within stimulated granules while in NS granules (intact) the labeling was mostly observed at the granule limiting membrane. (C) The total granule area as well as the CD63‐immunolabeled area increased in response to stimulation (*; #P < 0.05 vs. NS). In (D), the variation of CD63‐immunolabeled area in specific granules is shown in different groups. Eosinophils were isolated from the peripheral blood, stimulated or not with CCL11 or TNF‐α and prepared for pre‐embedding immunanogold EM. A total of 175 secretory (specific) granules showing pools of CD63 from CCL11‐stimulated or TNF‐α–stimulated cells and controls (n = 29 cells) were analyzed for area quantification. Scale bar, 600 nm. Data represent means ± sem.
Having not found a clear difference in the content of CD63 between unstimulated and stimulated eosinophils, we next evaluated the level of CD63 labeling on secretory granules. We evaluated whether granules undergoing degranulation had more labeling compared with unstimulated (intact) granules. Our EM protocol uses very small gold particles (1.4 nm in diameter) covalently conjugated with Fab fragments, which are only one‐third the size of a whole IgG molecule. These very small probes improve Ab penetration and provide more quantitative labeling of antigenic sites [28]. Considering that each Fab′ binds to one molecule of the antigen, quantification of the number of gold particles/granule would be informative. However, considering the intense immunolabeling in many secretory granules (Fig. 4), particle individualization was not always possible. We then evaluated the total granule area and the CD63‐immunolabeled area in each granule. Our results showed that although the area labeled for CD63 corresponded to a mean ± sem of 10.4 ± 7.6 nm2 per granule in unstimulated cells, the CD63‐labeled area was 70.5 ± 37.9 nm2 and 65.5 ± 25.7 nm2 (means ± sem) per granule for CCL11 and TNF‐α–stimulated cells, respectively (Fig. 6B and C). Moreover, in scoring the numbers of granules that exhibited CD63 labeling, in unstimulated cells, almost 100% of granules had an immunolabeled area <50 nm2/granule, whereas both stimuli elicited a marked increase of intragranular labeling, such that ∼60% of granules had a CD63+ area >50 nm2/granule (Fig. 6D). Individual granules exhibited an area up to 300 nm2 labeled for CD63, which corresponds to >50% of the total granule area (Fig. 6C and D) in stimulated cells. Of interest, granules undergoing release of their contents also showed an increase in their total area, that is, they were enlarged compared with intact granules (Fig. 6C).
Taken together, our data demonstrate that CD63 is highly associated with secretory events and is concentrated within granules undergoing active processes of secretion.
Large tubular carriers are involved in CD63 translocation
In this study, the presence of CD63‐labeled EoSVs was noted in the cytoplasm of both unstimulated (Fig. 3Bi) and stimulated eosinophils (Supplemental Fig. 2 and Fig. 7A and B ). Previous works from our group clearly showed that these vesicles associate with secretory granules, transport granule‐derived products, and increase in number in response to cell activation [15, 31], and then we evaluated whether the numbers of EoSVs changed after stimulation. Indeed, both stimuli induced significant formation of EoSVs compared with control cells (Fig. 7C). Not only did the total numbers of EoSVs increase but also the numbers of CD63+ EoSVs increased after stimulation with both agonists (Fig. 7C). Interestingly, many EoSVs were seen contacting granules undergoing secretion by PMD, as previously demonstrated [31], as well as by compound exocytosis (Fig. 7A, B, and D). We further investigated whether CD63+ vesicles had a differential distribution in the cytoplasm of eosinophils undergoing compound exocytosis. For this, we quantitated the numbers of CD63+ EoSVs per cytoplasm region, as we did for secretory granules. Remarkably, most CD63+ EoSVs in the cytoplasm of TNF‐α–stimulated cells were localized in the cell periphery ( Figs. 7E and 8 ), in association with more CD63+ granules in this region (Figs. 5B and 8). EoSVs labeled for CD63 were clearly fused with these granules (Figs. 7B and 8). Our data strongly indicate that tubular vesicles are acting in the translocation of CD63 from and to intracellular compartments, particularly, secretory granules, in response to stimulation.
Figure 7.
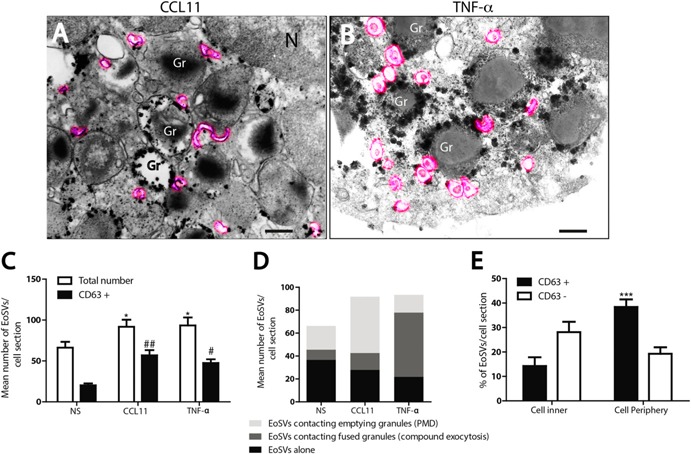
Vesicular trafficking of CD63 within human, stimulated eosinophils. (A and B) EoSVs (highlighted in pink) were observed in the cytoplasm surrounding or fused with secretory granules (Gr) within CCL11‐stimulated (A) or TNF‐α–stimulated cells. (C) Quantitative analyses of EoSV numbers. Note that, after stimulation, not only did the total number of EoSVs increase but also the number of CD63+ EoSVs. (D) Many EoSVs were seen contacting granules undergoing secretion. (E) After stimulation with TNF‐α, most CD63+ EoSVs were observed in the cell periphery. Eosinophils were isolated from the peripheral blood, stimulated or not with CCL11 or TNF‐α and prepared for pre‐embedding immunanogold EM. A total of 23 electron micrographs from unstimulated and stimulated cells were evaluated, and the numbers of labeled and not labeled EoSVs (n = 1945) were counted in each cell section. NS, not stimulated. *P < 0.05 vs. NS group (total EoSVs number); # P < 0.05 vs. NS group (CD63+ EoSVs); ##P < 0.01 vs. NS group (CD63+ EoSVs); ***P < 0.001 vs. CD63+ EoSVs at cell inner. Scale bar, 437 nm (A and Ai). Data represent means ± sem.
Figure 8.

CD63 is translocated on EoSVs to or from secretory granules after stimulation with TNF‐α. (A and Ai) A representative electron micrograph from an entire eosinophil profile shows CD63‐labeled EoSVs (highlighted in pink), mostly at cell periphery, in association with CD63+ secretory granules (Gr). The boxed area in (A) is shown in higher magnification in (Ai). Eosinophils were isolated from peripheral blood, stimulated with TNF‐α, and prepared for pre‐embedding immunanogold EM. N, nucleus. Scale bar, 950 nm (A), 630 nm (Ai).
DISCUSSION
The secretory responses of the specific granules of eosinophils, including their “degranulation,” underlie eosinophil responses to inflammatory, allergic, and immunoregulatory situations. Here, we identified, for the first time, to our knowledge, that a major intracellular pool of CD63 is directly coupled to degranulation events of human eosinophils, specifically PMD and compound exocytosis. We demonstrated that, in response to stimulation, CD63 is localized within granules undergoing losses of their contents and that CD63 traffics with both transport vesicles and granule movements in the cytoplasm. Thus, we recognized active, intracellular trafficking of CD63 linked to the eosinophil secretory pathway.
To induce different modes of secretion in eosinophils, we stimulated the cells with CCL11 or TNF‐α, which clearly elicited morphologic changes characteristic of PMD or compound exocytosis, respectively (Figs. 2 and 4). Both stimuli are known to promote eosinophil activation and release of products stored within secretory granules [23, 24, 26, 27, 30]. For example, the proinflammatory cytokine TNF‐α proved to be a potent stimulus, eliciting secretions of IL‐4, IL‐6, and INF‐γ from human eosinophils [27]. In fact, TNF‐α was shown to be essential for INF‐γ–induced secretion of Th1‐type chemokines and to enhance IL‐4–induced secretion of Th2‐type chemokines by human eosinophils [26]. CCL11 stimulation of human eosinophils led to specific release of IL‐4 [24]. Moreover, our group demonstrated that human eosinophil granules express functional CCR3 chemokine receptors [18] and secrete eosinophil cationic protein in response to CCL11 [18, 35].
Stimulation of human eosinophils with CCL11 or TNF‐α led to cell surface up‐regulation of CD63 (Fig. 1B and C and Supplemental Fig. 3). In contrast, Stubbs et al. [36] did not find CD63 expression at the eosinophil surface after stimulation with CCL11. This discrepancy with our results might be explained by the use of different times of stimulation or by the use of mixed‐cell suspensions (eosinophils and neutrophils) instead of purified eosinophils [36]. On the other hand, our results are in accord with previous studies using other agonists considered as inducers of PMD, such as INF‐γ [17] or platelet‐activating factor [14], which were able to cause up‐regulation of CD63 on human eosinophils [5, 17].
In fact, CD63 has been suggested to be involved with leukocyte secretory processes. For example, in human eosinophils, CD63 appeared to shift to the cell periphery after stimulation with INF‐γ, potentially linking CD63 with PMD and providing initial evidence for CD63 translocation in the cytoplasm [17]. On the other hand, a study on histamine release in stimulated human basophils has associated CD63 expression with the compound exocytosis form of secretion [21]. However, because these studies used fluorescence microscopy and precise visualization of secretory processes is possible only at high resolution by TEM, a direct link between CD63 and the secretory pathway in leukocytes has remained elusive.
Here, we provided a comprehensive investigation of CD63 at the EM level. Our study, using an immunonanogold EM technique that combines both sensitive antigen detection and detailed information on the cell structure [28], revealed that CD63 is tightly associated not only with PMD but also with compound exocytosis. A notable, ultrastructural observation was that CD63 traffics in the cytoplasm in concert with the movement of granules involved in compound exocytosis (Figs. 4B and 5). CD63 may be acting as a secretion facilitator/regulator molecule, or it may have a more‐direct role in the intracellular transport of granule‐derived products. Further experiments, including dual‐localization studies of CD63 and specific granule‐stored products, will be required to explore this possibility.
One interesting observation from the present study was that although CD63 was observed at the eosinophil's cell surface after stimulation (Fig. 1B and C), a robust pool of CD63 seems to remain in the cytoplasm, as observed by immunonanogold EM (Fig. 4). This technique revealed that after 1 h of agonist stimulation, when a consistent loss of granule content was detected (Fig. 2), CD63 was still strongly observed in association with secretory granules (Fig. 4). Thus, we can conclude that a strong, intracellular pool of CD63 is implicated in the eosinophil secretory pathway and that most of this internal CD63 is not completely externalized in response to stimulation. By using flow cytometry, it was also demonstrated in human eosinophils that the CD63 expression at the cell surface increased after agonist stimulation (10 min), although the total intracellular CD63 expression was similar in resting and agonist‐stimulated cells [17].
Our Western blotting results did not detect a difference in the CD63 total content when unstimulated and stimulated cells were compared (Fig. 6A). However, by EM, we observed that stimulated eosinophils had more CD63+ granules (∼20%) (Fig. 3A). In addition, we demonstrated that CD63 was more‐prominently detectable in granules in the process of secretion. Of note, our EM analyses were focused on specific granules, whereas Western blotting quantitated the total CD63 expression, which can be found in other compartments besides granules. The many CD63+ granules in both unstimulated and stimulated eosinophils and the Western blotting results indicate that CD63 is present as a robust, preformed pool in human eosinophils.
Our finding that pools of CD63 traffic between intracellular compartments raises the question how CD63—a transmembrane molecule—is transported in the cytoplasm. One possibility is that secretory granules in the process of content release acquire CD63 from membrane‐bound transport carriers. Indeed, a remarkable finding was the marked increase of CD63+ EoSVs in stimulated cells. Additionally, the cytoplasmic distribution of these vesicles changed after TNF‐α stimulation, increasing in number at the cell periphery around fused granules. Thus, it is likely that EoSVs, which act in the transport of eosinophil granule cargos [37], are involved in the CD63 mobilization and transfer to secretory granules after fusion with these organelles, as frequently observed (Figs. 7B and 8). Accumulation of this tetraspanin within these organelles may be explained by the presence of intragranular membranes, which constitute an elaborate tubular network able to sequester and relocate granule products upon stimulation of human eosinophils [14]. The presence of CD63+ membranes has been recognized not only within eosinophil secretory granules [14] but also in other lysosome‐related organelles, such as platelet‐α granules [38] and MHC class II compartments in dendritic cells [39].
Taken together, our findings demonstrate that an intracellular CD63 trafficking is consistently connected to the secretory pathway of human eosinophils and is likely participating in the processes of release of granule‐stored products.
AUTHORSHIP
R.C.N.M. provided the study conception and design. P.F.W. and R.C.N.M. provided study guidance, mentorship, and critical editing of the manuscript. L.A.S.C., K.B., S.U., J.S.N., L.L., L.A.S., and R.C.N.M. performed experiments and acquired and analyzed the data. L.A.S.C., L.A.S., A.M.D., P.F.W., and R.C.N.M. interpreted data. L.A.S.C. and R.C.N.M. prepared the manuscript. P.F.W. and R.C.N.M. share senior authorship of this work. All authors contributed in part to writing and editing the manuscript and approved the final version.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases Grants USA‐R37AI020241 and R01AI022571; NIH National Heart, Lung, and Blood Institute Grant R01HL095699; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Grants Brazil477475/2013‐2, 469995/2014‐9, and 311083/2014‐5; and the Brazilian Ministry of Health and Fundacão de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) Grant Brazil‐CBB‐APQ‐02239‐14. The authors gratefully acknowledge the skillful assistance of Ellen Morgan (Electron Microscopy Unit, Department of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School).
Supporting information
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4
References
- 1. Stow, J. L. , Murray, R. Z. (2013) Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 24, 227–239. [DOI] [PubMed] [Google Scholar]
- 2. Spencer, L. A. , Bonjour, K. , Melo, R. C. , Weller, P. F. (2014) Eosinophil secretion of granule‐derived cytokines. Front. Immunol. 5, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melo, R. C. N. , Dvorak, A. M. , Weller, P. F. 2012. Eosinophil ultrastructure In Eosinophils in health and disease. (Lee J. R., Rosenberg H., eds.), Vol. 1 Elsevier, New York, p. 20–27. [Google Scholar]
- 4. McLaren, D. J. , Mackenzie, C. D. , Ramalho‐Pinto, F. J. (1977) Ultrastructural observations on the in vitro interaction between rat eosinophils and some parasitic helminths (Schistosoma mansoni, Trichinella spiralis and Nippostrongylus brasiliensis). Clin. Exp. Immunol. 30, 105–118. [PMC free article] [PubMed] [Google Scholar]
- 5. Inoue, Y. , Matsuwaki, Y. , Shin, S. H. , Ponikau, J. U. , Kita, H. (2005) Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J. Immunol. 175, 5439–5447. [DOI] [PubMed] [Google Scholar]
- 6. Karawajczyk, M. , Sevéus, L. , Garcia, R. , Björnsson, E. , Peterson, C. G. , Roomans, G. M. , Venge, P. (2000) Piecemeal degranulation of peripheral blood eosinophils: a study of allergic subjects during and out of the pollen season. Am. J. Respir. Cell Mol. Biol. 23, 521–529. [DOI] [PubMed] [Google Scholar]
- 7. Erjefält, J. S. , Greiff, L. , Andersson, M. , Adelroth, E. , Jeffery, P. K. , Persson, C. G. (2001) Degranulation patterns of eosinophil granulocytes as determinants of eosinophil driven disease. Thorax 56, 341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahlstrom‐Emanuelsson, C. A. , Greiff, L. , Andersson, M. , Persson, C. G. , Erjefält, J. S. (2004) Eosinophil degranulation status in allergic rhinitis: observations before and during seasonal allergen exposure. Eur. Respir. J. 24, 750–757. [DOI] [PubMed] [Google Scholar]
- 9. Cheng, J. F. , Ott, N. L. , Peterson, E. A. , George, T. J. , Hukee, M. J. , Gleich, G. J. , Leiferman, K. M. (1997) Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J. Allergy Clin. Immunol. 99, 683–692. [DOI] [PubMed] [Google Scholar]
- 10. Caruso, R. A. , Ieni, A. , Fedele, F. , Zuccalà, V. , Riccardo, M. , Parisi, E. , Parisi, A. (2005) Degranulation patterns of eosinophils in advanced gastric carcinoma: an electron microscopic study. Ultrastruct. Pathol. 29, 29–36. [DOI] [PubMed] [Google Scholar]
- 11. Raqib, R. , Moly, P. K. , Sarker, P. , Qadri, F. , Alam, N. H. , Mathan, M. , Andersson, J. (2003) Persistence of mucosal mast cells and eosinophils in Shigella‐infected children. Infect. Immun. 71, 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qadri, F. , Bhuiyan, T. R. , Dutta, K. K. , Raqib, R. , Alam, M. S. , Alam, N. H. , Svennerholm, A. M. , Mathan, M. M. (2004) Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut 53, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Melo, R. C. N. , Weller, P. F. (2010) Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol. Histopathol. 25, 1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melo, R. C. N. , Perez, S. A. , Spencer, L. A. , Dvorak, A. M. , Weller, P. F. (2005) Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic 6, 866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melo, R. C. N. , Spencer, L. A. , Perez, S. A. , Neves, J. S. , Bafford, S. P. , Morgan, E. S. , Dvorak, A. M. , Weller, P. F. (2009) Vesicle‐mediated secretion of human eosinophil granule‐derived major basic protein. Lab. Invest. 89, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pols, M. S. , Klumperman, J. (2009) Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 315, 1584–1592. [DOI] [PubMed] [Google Scholar]
- 17. Mahmudi‐Azer, S. , Downey, G. P. , Moqbel, R. (2002) Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood 99, 4039–4047. [DOI] [PubMed] [Google Scholar]
- 18. Neves, J. S. , Perez, S. A. , Spencer, L. A. , Melo, R. C. N. , Reynolds, L. , Ghiran, I. , Mahmudi‐Azer, S. , Odemuyiwa, S. O. , Dvorak, A. M. , Moqbel, R. , Weller, P. F. (2008) Eosinophil granules function extracellularly as receptor‐mediated secretory organelles. Proc. Natl. Acad. Sci. U. S. A. 105, 18478–18483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim, J. D. , Willetts, L. , Ochkur, S. , Srivastava, N. , Hamburg, R. , Shayeganpour, A. , Seabra, M. C. , Lee, J. J. , Moqbel, R. , Lacy, P. (2013) An essential role for Rab27a GTPase in eosinophil exocytosis. J. Leukoc. Biol. 94, 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pak, V. , Budikhina, A. , Pashenkov, M. , Pinegin, B. (2007) Neutrophil activity in chronic granulomatous disease. Adv. Exp. Med. Biol. 601, 69–74. [DOI] [PubMed] [Google Scholar]
- 21. MacGlashan, D., Jr. , (2010) Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin. Exp. Allergy 40, 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pfistershammer, K. , Majdic, O. , Stöckl, J. , Zlabinger, G. , Kirchberger, S. , Steinberger, P. , Knapp, W. (2004) CD63 as an activation‐linked T cell costimulatory element. J. Immunol. 173, 6000–6008. [DOI] [PubMed] [Google Scholar]
- 23. Egesten, A. , Blom, M. , Calafat, J. , Janssen, H. , Knol, E. F. (1998) Eosinophil granulocyte interaction with serum‐opsonized particles: binding and degranulation are enhanced by tumor necrosis factor alpha. Int. Arch. Allergy Immunol. 115, 121–128. [DOI] [PubMed] [Google Scholar]
- 24. Bandeira‐Melo, C. , Sugiyama, K. , Woods, L. J. , Weller, P. F. (2001) Cutting edge: eotaxin elicits rapid vesicular transport‐mediated release of preformed IL‐4 from human eosinophils. J. Immunol. 166, 4813–4817. [DOI] [PubMed] [Google Scholar]
- 25. Bandeira‐Melo, C. , Perez, S. A. , Melo, R. C. N. , Ghiran, I. , Weller, P. F. (2003) EliCell assay for the detection of released cytokines from eosinophils. J. Immunol. Methods 276, 227–237. [DOI] [PubMed] [Google Scholar]
- 26. Liu, L. Y. , Bates, M. E. , Jarjour, N. N. , Busse, W. W. , Bertics, P. J. , Kelly, E. A. (2007) Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF‐α. J. Immunol. 179, 4840–4848. [DOI] [PubMed] [Google Scholar]
- 27. Spencer, L. A. , Szela, C. T. , Perez, S. A. , Kirchhoffer, C. L. , Neves, J. S. , Radke, A. L. , Weller, P. F. (2009) Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 85, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melo, R. C. N. , Morgan, E. , Monahan‐Earley, R. , Dvorak, A. M. , Weller, P. F. (2014) Pre‐embedding immunogold labeling to optimize protein localization at subcellular compartments and membrane microdomains of leukocytes. Nat. Protoc. 9, 2382–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bandeira‐Melo, C. , Gillard, G. , Ghiran, I. , Weller, P. F. (2000) EliCell: a gel‐phase dual antibody capture and detection assay to measure cytokine release from eosinophils. J. Immunol. Methods 244, 105–115. [DOI] [PubMed] [Google Scholar]
- 30. Carmo, L. A. , Dias, F. F. , Malta, K. K. , Amaral, K. B. , Shamri, R. , Weller, P. F. , Melo, R. C. N. (2015) Expression and subcellular localization of the Qa‐SNARE syntaxin17 in human eosinophils. Exp. Cell Res. 337, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Melo, R. C. N. , Spencer, L. A. , Perez, S. A. , Ghiran, I. , Dvorak, A. M. , Weller, P. F. (2005) Human eosinophils secrete preformed, granule‐stored interleukin‐4 through distinct vesicular compartments. Traffic 6, 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dias, F. F. , Amaral, K. B. , Carmo, L. A. , Shamri, R. , Dvorak, A. M. , Weller, P. F. , Melo, R. C. N. (2014) Human eosinophil leukocytes express protein disulfide isomerase in secretory granules and vesicles: ultrastructural studies. J. Histochem. Cytochem. 62, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lintomen, L. , Franchi, G. , Nowill, A. , Condino‐Neto, A. , de Nucci, G. , Zanesco, A. , Antunes, E. (2008) Human eosinophil adhesion and degranulation stimulated with eotaxin and RANTES in vitro: lack of interaction with nitric oxide. BMC Pulm. Med. 8, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Willetts, L. , Ochkur, S. I. , Jacobsen, E. A. , Lee, J. J. , Lacy, P. (2014) Eosinophil shape change and secretion. Methods Mol. Biol. 1178, 111–128. [DOI] [PubMed] [Google Scholar]
- 35. Ueki, S. , Melo, R. C. N. , Ghiran, I. , Spencer, L. A. , Dvorak, A. M. , Weller, P. F. (2013) Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion‐competent eosinophil granules in humans. Blood 121, 2074–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stubbs, V. E. , Schratl, P. , Hartnell, A. , Williams, T. J. , Peskar, B. A. , Heinemann, A. , Sabroe, I. (2002) Indomethacin causes prostaglandin D2‐like and eotaxin‐like selective responses in eosinophils and basophils. J. Biol. Chem. 277, 26012–26020. [DOI] [PubMed] [Google Scholar]
- 37. Melo, R. C. N. , Spencer, L. A. , Dvorak, A. M. , Weller, P. F. (2008) Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule‐derived cytokines and other proteins. J. Leukoc. Biol. 83, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heijnen, H. F. , Debili, N. , Vainchencker, W. , Breton‐Gorius, J. , Geuze, H. J. , Sixma, J. J. (1998) Multivesicular bodies are an intermediate stage in the formation of platelet alpha‐granules. Blood 91, 2313–2325. [PubMed] [Google Scholar]
- 39. Barois, N. , de Saint‐Vis, B. , Lebecque, S. , Geuze, H. J. , Kleijmeer, M. J. (2002) MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic 3, 894–905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4


