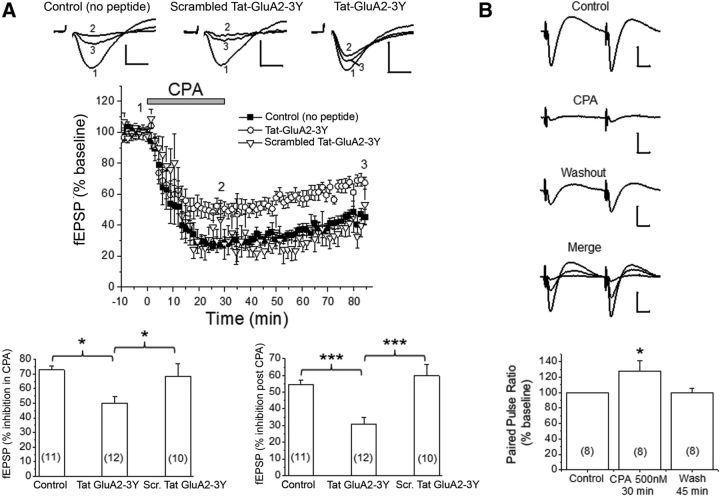
Figure 4.
Prolonged stimulation of A1Rs caused A1R-induced persistent synaptic depression (APSD) in part via clathrin-mediated GluA2 internalization. A, Top, Representative fEPSP traces from hippocampal CA1 region in the absence of Tat-peptides (Control), in scrambled Tat-GluA2–3Y peptide (2 μm), and in Tat-GluA2–3Y peptide (2 μm). The numbers associated with the fEPSP traces correspond to baseline control (1), 30 min after CPA application (2), and 55 min after CPA washout (3), and also apply to middle panel. The time course of CPA-induced synaptic depression is summarized in the middle panel, showing that Tat-GluA2–3Y peptide, but not its scrambled version, partially inhibited the CPA-induced APSD. Bottom, Control (no peptide) and scrambled Tat-GluA2–3Y produced similar levels of synaptic depression during CPA application (left) and after CPA washout (right), whereas the Tat-GluA2–3Y peptide significantly attenuated these responses. *p < 0.05 versus Control (no peptide) or scrambled Tat-GluA2–3Y (Student-Neuman-Keuls post hoc test). ***p < 0.001 versus Control (no peptide) or scrambled Tat-GluA2–3Y (Student-Neuman-Keuls post hoc test). B, Paired-pulse stimulation shows that synaptic depression during CPA was accompanied by significant paired pulse facilitation (bottom panel, *p < 0.05 vs Control), but synaptic depression during APSD showed paired pulse ratios similar to baseline control levels. Numbers inside summary bar charts refer to the number of brain slices from different animals. Data are mean ± SEM. Vertical calibration: 0.5 mV. Horizontal calibration: A, 5 ms; B, 10 ms. These results indicate a functional interaction between A1Rs and AMPARs, leading to clathrin-mediated internalization of GluA2-containing AMPARs and subsequent induction of APSDs.