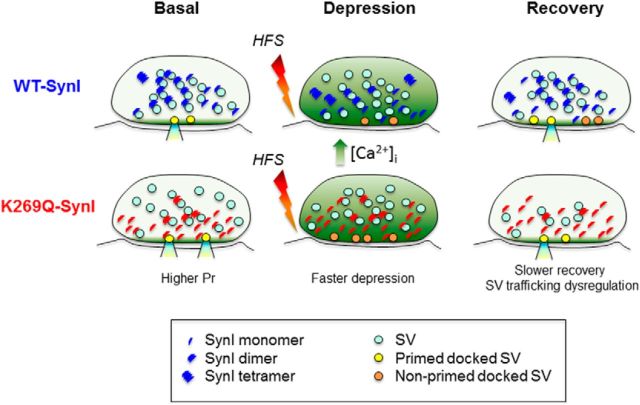
Figure 8.
Mechanistic diagram of the SV dysregulation in the absence of ATP binding to SynI. An inhibitory nerve terminal expressing either WT-SynI (blue symbols; top row) or K269Q-SynI (red symbols; bottom row) is shown under basal conditions (left), at the end of a sustained high-frequency stimulation (HFS; 30 s at 10 Hz; middle) and over recovery from depression (right). The oligomerization state of SynI has been hypothesized on the basis of the data of Figure 3. Under basal conditions, Pr is higher in mutant terminals (Fig. 5) and SVs are more dispersed due to the decreased SV binding of SynI (Fig. 2) and the relative decrease of high-order SynI oligomers (Fig. 3). Accordingly, the mutant SynI accumulates in proximity to the presynaptic membrane (Fig. 4), possibly contributing to the increase in Pr. After sustained HFS, mutant terminals display a precocious increase in nonprimed docked SVs possibly resulting from the increased SV availability and a concomitant decrease in Pr (Figs. 6, 7). After depression, mutant terminals recover more slowly but completely at the expense of a transient depletion of total SVs (Figs. 6, 7). The increase in the formation of SynI tetramers versus dimers upon ATP and Ca2+ binding in WT terminals and the parallel reduction in SV clustering may represent a mechanism to increase SV availability under conditions of sustained stimulation associated with Ca2+ build-up.