Figure 1.
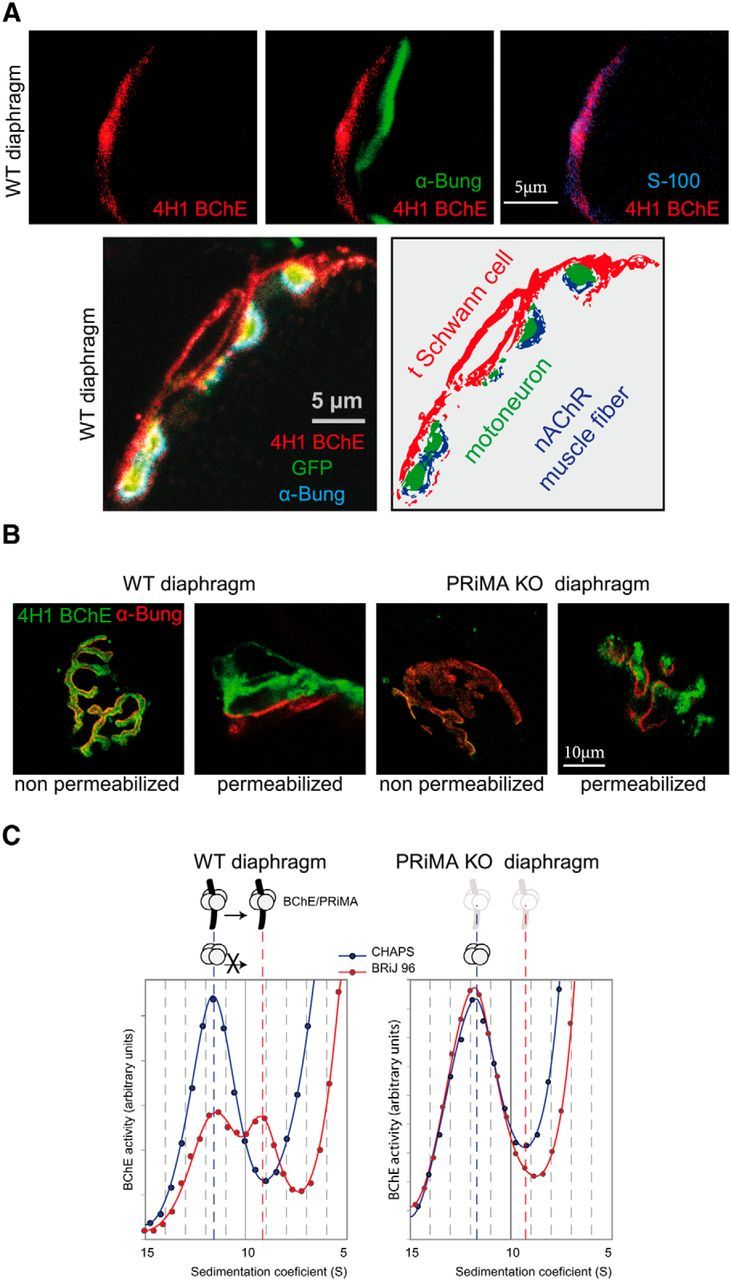
BChE is localized at the TSC with PRiMA. A, Top, BChE was labeled with biotinylated 4H1 (red), the muscle nAChR localized on the top of the fold at the surface were labeled with α-bungarotoxin (green), and S100 a specific marker of the TSCs were labeled with specific antibodies (blue). The overlay of BChE/α-bungarotoxin labeling (middle) and of BChE/S100 labeling (right) shows that BChE is mainly localized at the TSC. Bottom, Immunolocalization with biotinylated 4H1 (red) in a mouse strain in which the motoneuron are labeled by membrane tethered GFP (green). The muscle nAChR are labeled with α-bungarotoxin (blue). The drawing illustrates the localization of BChE on the surface of the TSC. B, Labeling of BChE with biotinylated 4H1 (green) and of muscle nAChR with α-bungarotoxin (red) in muscle diaphragm of WT and PRiMA KO mice. Nonpermeabilized indicates that the muscle was incubated in toto without permeabilization before the application of antibodies and toxin. Note the absence of BChE labeling in PRiMA KO mice. Permeabilized indicates that frozen sections of muscle were incubated with antibodies and toxins. Note the presence of BChE in WT and PRiMA KO mice. The labeling in PRiMA KO mice appears more spotted than in WT mice. Altogether, these images show that BChE is mainly anchored by PRiMA at the surface of the TSC at the NMJ. C, Two BChE tetramers are found in the diaphragm. Enzymes were solubilized and loaded in sucrose gradients containing CHAPS or Brij as the detergent. After centrifugation, BChE activity was determined in all of the fractions. The figure presents a part of the gradient around the peaks of BChE activity that correspond to the tetramer. In the CHAPS gradient, the peak at 11.5 S corresponds to the tetramers. Amphiphilic tetramer (interaction with detergent) shifts in the presence of Brij and is absent in PRiMA KO mice, demonstrating that some of the tetramers of BChE are anchored by PRiMA.
