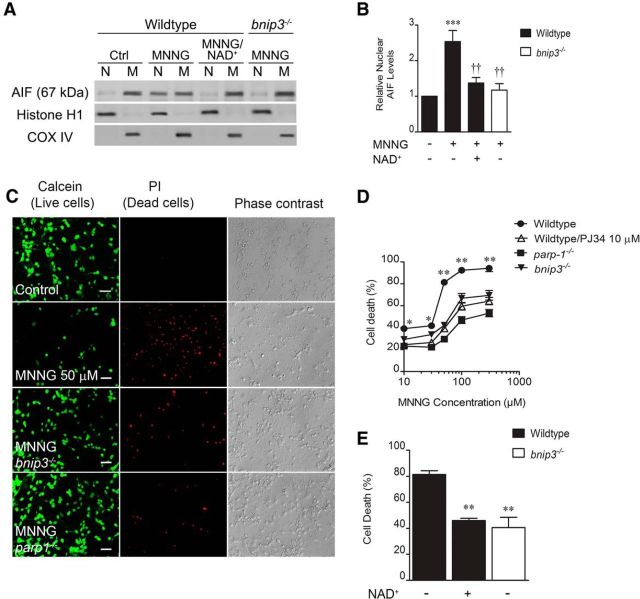
Figure 3.
PARP1 causes Bnip3-dependent AIF translocation and neuron death. A, Cortical neurons were exposed to MNNG (50 μm, 30 min). Mitochondrial and nuclear fractions were assessed for immunoreactivity for AIF 4 h after MNNG removal. B, MNNG significantly increased nuclear AIF in a manner reduced by NAD+ supplementation (1 mm) or Bnip3 deletion. ***p < 0.001, compared with 0 MNNG control; ††p < 0.01, compared with the wild-type, MNNG-treated group. Data were analyzed using ANOVA with the Student–Newman–Keuls post hoc test. C, Red, MNNG also caused Bnip3-dependent cell death. Green cells are live and accumulate calcein, and the third column shows phase contrast images. D, MNNG-induced neuron death was dose dependent, and was reduced by PJ34 (10 μm) and the deletion of either PARP1 or Bnip3. *p < 0.05, **p < 0.01, compared with all other groups at the same time point. Data were analyzed using two-way ANOVA with the Bonferroni's post hoc test. E, Eighteen hours after MNNG (50 μm, 30 min), both Bnip3 deletion and NAD+ supplementation significantly reduced neuron death. **p < 0.01, compared with the wild-type, MNNG-treated group. Data were analyzed using ANOVA with the Student–Newman–Keuls post hoc test. All data are reported as the mean ± SEM (n = 5–7). Scale bar, 40 μm.