Abstract
Our memories form a record not only of our experiences, but also of their temporal structure. Although memory for the temporal structure of experience likely relies on multiple neural systems, numerous studies have implicated the hippocampus in the encoding and retrieval of temporal information. This review evaluates the literature on hippocampal contributions to human serial-order memory from the perspective of three cognitive theories: associative chaining theory, positional-coding theory and retrieved-context theory. Evaluating neural findings through the lens of cognitive theories enables us to draw more incisive conclusions about the relations between brain and behavior.
Keywords: episodic memory, fMRI, iEEG, sequence memory, temporal order
1 |. INTRODUCTION
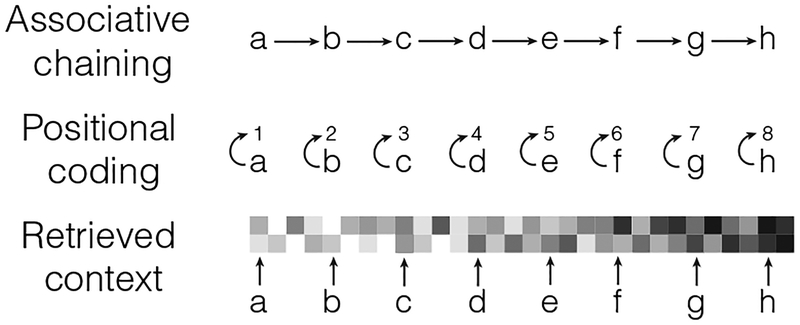
Knowing the order of events is critical for many aspects of daily life. Investigations of the neural correlates of memory in both humans and animals have implicated the hippocampus in supporting this vital ability (Manns, Howard, & Eichenbaum, 2007; Davachi & DuBrow, 2015). Far less clear, however, is exactly how the hippocampus accomplishes this function. In this review, we take stock of the current state of the literature on hippocampal contributions to the encoding and retrieval of serial-order information. We relate these neural investigations to three cognitive theories of sequence memory: associative chaining theory, positional coding theory, and retrieved-context theory (Figure 1).
FIGURE 1.
Theories of serial-order memory. Simplified illustration of three major classes of models. In associative chaining, associations are formed directly between study items. In positional coding, associations are formed between items and their serial position. In retrieved-context theory, items are associated to a slowly updating context representation
According to associative chaining theory, the memory system directly links the representations of successively experienced events (e.g., study items in a list learning task). Subsequent presentation or recall of a given item cues recall of the contiguously presented items (Ebbinghaus, 1885/1913; Lewandowsky & Murdock, 1989; DuBrow & Davachi, 2013). Proposed by Ebbinghaus (1885/1913), direct item–item associations can account for a number of behavioral findings, including error patterns in serial recall (Solway, Murdock, & Kahana, 2012; Franklin & Mewhort, 2015) and performance decrements on lists in which study items vary along a specific dimension (Morin, Poirier, Fortin, & Hulme, 2006; Caplan, 2015). Specifically, after recalling an item out of sequence, subjects are likely to transition to neighboring study items (Solway et al., 2012), suggesting that items themselves are being used as retrieval cues. Likewise, performance is worse for “mixed” lists in which words alternate in frequency compared with “pure” lists of only high or only low frequency (Caplan, 2015), again suggesting that direct item–item associations drive serial-order memory.
In contrast to associative chaining, the positional-coding theory posits that the memory system links each item with a representation of its time of occurrence, or its position within a series (Yntema & Trask, 1963; Conrad, 1965; Friedman, 1993; Henson, 1998; Brown, Preece, & Hulme, 2000; Botvinick & Plaut, 2006). This position is then used as a cue to retrieve the item to which it was associated. This theory was developed and has been used to account for a number of behavioral findings that are inconsistent with the associative chaining theory. For instance, according to chaining theory, studying a list of sequentially presented items should lead to the formation of associations between neighboring items. If those same items are then divided into pairs for a paired associates task, the existence of direct item–item associations should “transfer,” facilitating performance. However, researchers failed to observe a transfer effect (Young, 1962) and proposed instead that associations are formed between items and positions, an association which would not necessarily facilitate paired associates learning.
Finally, retrieved-context theories of memory assert that items become associated with a spatiotemporal contextual representation, analogous to positional or temporal codes. However, according to this account, recall of an item reinstates its temporal context, which in turn, cues subsequent retrievals (Anderson & Bower, 1972; Howard & Kahana, 2002; Polyn, Norman, & Kahana, 2009a; Lohnas, Polyn, & Kahana, 2015). This theory may be viewed as a hybrid between positional coding and associative chaining theories (Caplan, 2015) in that the spatiotemporal contextual representation serves to bind contiguously studied items. According to retrieved-context theory, items become bidrectionally associated with a representation of spatiotemporal context during both memory encoding and retrieval. Further, the activation of item features during either encoding or retrieval results in reactivation of previously associated contexts. These reactivated contexts, in turn, combine with the currently active context representation to form a new context (Polyn & Kahana, 2008).
Within the laboratory, researchers have used a variety of paradigms to test these theories of serial-order memory. Serial-order memory mechanisms are thought to drive behavioral phenomena both in serial learning tasks that explicitly require subjects to serially recall memoranda as well as in tasks without such a requirement. Explicit serial order tasks include serial learning, immediate serial recall, judgments of recency, and serial reordering. In these paradigms, subjects deliberately attempt to learn and subsequently recall or recognize an ordered sequence of items. For example, in an immediate serial recall paradigm, subjects study a series of individually presented items and then attempt to recall the studied items in forward serial order. In a judgment of recency task, after studying a series of items, subjects judge the order of a pair of test items, indicating which item appeared more recently. Serial-order memory mechanisms can likewise be understood via tasks that implicitly leverage serial order information. Implicit tasks include the serial-response-time procedure, in which subjects simply make judgments on each item in a long series and items repeat in a predictable order. Researchers infer knowledge of serial order information from subjects’ improved performance on items. Knowledge of serial order information can also be inferred in free recall tasks, where subjects choose to recall items in serial order even when this is not required by the task.
2 |. THE HIPPOCAMPUS AND SERIAL-ORDER MEMORY
There is considerable evidence that the hippocampus supports complex forms of associative memory; the hippocampus shows increased activity during the formation and retrieval of associations (Eichenbaum, 2017; Suzuki, 2007; Fortin, Agster, & Eichenbaum, 2002; Davachi, Mitchell, & Wagner, 2003; Ranganath et al., 2004), and hippocampal lesions impair the ability to encode and retrieve associations (Kesner, Gilbert, & Barua, 2002; Fortin et al., 2002; Farovik, Dupont, & Eichenbaum, 2010). However, these results do not clarify the mechanisms by which the hippocampus supports these associations. The goal of this review is to survey human intracranial electroencephalographic (iEEG) and functional magnetic resonance imaging (fMRI) data recorded while subjects engage in tasks thought to be supported by serial-order memory mechanisms. All three theories posit that an association formed during study is leveraged during retrieval. The critical question concerns the nature of these associations: does this hippocampus associate items to one another (associative chaining), to a position or temporal tag (positional coding), or some hybrid of the two, a spatiotemporal context representation (retrieved-context)?
fMRI investigations of hippocampal activity have found reliable increases in the hippocampus both during the encoding and the retrieval of serial order information (Konishi, Uchida, Machida, Shirouzu, & Miyashita, 2002; Ekstrom & Bookheimer, 2007; Lehn et al., 2009; Ekstrom, Copara, Isham, Wang, & Yonelinas, 2011; Tubridy & Davachi, 2011). Tubridy and Davachi (2011) had subjects study triplets of sequentially presented words and then reorder those items during test. The authors found that increased hippocampal activity during encoding predicted better performance on the subsequent ordering task. This result is most clearly accounted for by the positional coding theory. A stronger link between item and position during study, reflected by increased hippocampal activity, would facilitate the retrieval of position information at test, allowing subjects to correctly reorder each triplet. In comparison, a stronger link between items or between items and a gradually changing context representation would not necessarily facilitate a subsequent order judgment. Basic associative chaining models do not store directionality information (Lewandowsky & Murdock, 1989), which means that the ability to judge the order of triplets should be at chance were one to rely on direct item–item associations. Likewise, the context representation that would be associated to each item would be highly similar within a triplet. Unless this context representation contained explicit position information, the relative order of each item would not be discriminable.
Ultimately, in explicit serial order tasks, univariate signals alone are unlikely to be able to distinguish the particular type of association that supports performance. Although increased hippocampal activity suggests that associations are being formed, such activity in a serial recall task does not elucidate the type of association being formed. By combining multivariate pattern analysis with the serial recall task, it is possible to assess which form of associations support serial-order memory (Kalm, Davis, & Norris, 2013). Kalm et al. (2013) used multi-voxel pattern analysis to track hippocampal activity patterns as subjects learned sequences of letters across repeated study-test trials. The same eight consonants were used for every sequence, thus sequences could only be differentiated by using a combination of item (letter) and position information. The authors assessed similarity across repetitions of the same sequence and between different sequences. Multiple brain regions including the hippocampus showed increased pattern similarity across repetitions of the same sequence. However, the hippocampus was the only region to also show a concurrent decrease in pattern similarity across repetitions of different sequences.
Here we consider which of the three theories of serial-order memory best accounts for these findings. Once a sequence is learned, subjects may begin to predict upcoming items (Kumaran & Maguire, 2006b; Davachi & DuBrow, 2015). Using either position codes or items alone to generate such predictions should not lead to differentiation across sequences, as the same positions and the same items were used across sequences. However, if subjects are retrieving contextual representations associated with items, sequences will be differentiable as the context representations of the same item across two different sequences will differ. Consider the following two sequences: ABCDEF and DFCBAE. The context representation associated with C in List 1 consists of items A, B, and C whereas in List 2 it consists of D, F, and C. Thus, the neural signals associated with item C will differ across different sequences. This logic can be extended to all list items, meaning that sequences will be differentiable on account of the contextual information that is retrieved by each item. With repeated exposure, context reinstatement should become more robust, leading to greater differences in neural similarity across sequences.
There is corroborating evidence that the hippocampus represents contextual information (Ezzyat & Davachi, 2014). Ezzyat and Davachi (2014) had subjects study faces and objects paired with scenes and during test, subjects had to make a distance judgment about an object and a face. Although distance judgments do not necessarily reflect temporal order—one may know that “A” and “E” are far apart without knowing that A precedes E—the authors found that hippocampal activity tracked distance judgments. That is, greater hippocampal pattern similarity between two items at encoding predicted smaller subsequent distance judgments, even when the objective distance (and thus relevant position information) between two pairs of items was equal (e.g., “A” and “E” vs. “B” and “F”).
Given these results, one may expect that greater hippocampal pattern dissimilarity between two items should support relative recency judgments. Temporal distinctiveness models suggest that greater temporal distance between items facilitates memory for their relative order (Brown, Neath, & Chater, 2007). In turn, increased neural similarity between the encoding of two items should make them temporally confusable and consequently impede subjects’ ability to judge their relative recency. Jenkins & Ranganath (2016) confirmed this prediction in an fMRI study. Subjects encoded objects while performing a semantic judgment task; then, during test, subjects judged the relative recency of pairs of studied items. The authors found that greater encoding dissimilarity between pairs of items led to more accurate discrimination of relative recency.
In apparent contradiction to this result, DuBrow and Davachi (2014) found that greater hippocampal pattern similarity between pairs of encoding items predicted more accurate discrimination of relative recency. Subjects studied interleaved faces and objects, where switches in stimulus category (face or object) denoted a context boundary. A recency judgment was considered within boundary if both items were from a single category stream (e.g., two objects separated by other objects) and across boundary if the opposite category intervened between the two probes (e.g., two objects separated by faces). Hippocampal pattern similarity was greater for within compared to between boundary items. These results are most clearly accounted for by associative chaining whereby items are directly associated to one another. Multivariate results during retrieval further support this account (DuBrow & Davachi, 2014); the authors find evidence suggesting that intervening items are retrieved during recency judgments, consistent with the associative chaining prediction that direct item–item associations are leveraged during retrieval.
Whereas recency judgment tasks directly test subjects’ knowledge of the serial order of studied items, free recall tasks provide an indirect assay of subjects’ memory for serial order information. Although subjects may recall items in any order, they exhibit a marked tendency to sequentially recall items studied in neighboring input positions. This contiguity effect appears at diverse time scales and does not appear to be diminished when items are separated by distracting activity which suggests that contiguity more likely reflects some type of temporal coding process rather than a process based on interitem rehearsal, or direct item-to-item associations (Howard & Kahana, 1999, 2002).
Long and Kahana (2015) asked whether hippocampal activity measured during the encoding period of a free recall task would predict subjects’ tendency to transition among neighboring items during recall. They examined high frequency activity (HFA, 44–100 Hz) recorded from bipolar electrode pairs, as this signal correlates with both neuronal activity and the BOLD fMRI signal (Miller, Zanos, Fetz, den Nijs, & Ojemann, 2009; Mukamel et al., 2005), and is thought to be a marker of general activation within a brain region (Burke, Ramayya, & Kahana, 2015). Within the hippocampus, HFA reliably increased during the encoding of items that were later serially recalled compared with those items that were not serially recalled (but still remembered). An across-subject correlation showed that greater hippocampal activity during encoding predicted increased serial recall. In a followup study, Long et al. (2017) examined both hippocampal and neocortical HFA during the retrieval phase of the same free recall task. They divided recall events into three classes: recall of items from the immediately preceding list (correct recalls), recall of items from other study lists or items not studied (intrusions) and periods of searching with no recall event (deliberations). Regions supporting contextually mediated retrieval should show selective increases in activity preceding correct recalls only, as only those items are from the correct temporal context. In comparison, a general retrieval mechanism would be characterized by activity increases preceding both correct recalls and intrusions, as both classes are retrieval events. Long and colleagues found that HFA in the hippocampus was only reliably increased preceding correct recalls and that this effect was absent in neocortex.
On first pass this univariate data could seem to support any of the three theories as increased hippocampal HFA could reflect the formation and retrieval of either item–item, item–position, or item–context associations. However, given the behavioral and modeling evidence suggesting that contiguity in free recall is scale-invariant (Howard & Kahana, 1999; Shankar & Howard, 2012), the associative chaining theory is unlikely to account for these effects. Across longer delays and distractor periods, direct item associations become untenable, and yet, contiguity is still observed in these cases. Thus, these signals are more likely to reflect the presence of either item–position or item–context associations. Support specifically for retrieved-context theory is provided by recent evidence showing that incorporating hippocampal activity into context models provides a superior fit to behavior (Kragel, Morton, & Polyn, 2015).
Like free recall, sequence learning tasks provide an indirect measure of subjects’ memory for serial order information. However, unlike free recall, univariate investigations of sequence learning tasks can more clearly distinguish among the competing theories of serial-order memory. Subjects typically perform an incidental task (e.g., a one-back repetition task) and reaction time is used to infer memory for serial order. The hippocampus shows increased activation during sequence learning and retrieval (Schendan, Searl, Melrose, & Stern, 2003; Ross, Brown, & Stern, 2009) and often shows specific increases for non-identical, overlapping series of stimuli (Kumaran & Maguire, 2005, 2006a,b; Wang & Diana, 2016). For example, Kumaran and Maguire (2006b) had subjects view repeated, rearranged, or novel sequences. Object order was consistent across presentations of repeated sequences; however, for rearranged sequences, the third and fourth objects swapped serial position on the second presentation. The hippocampus showed reliably greater activity for these rearranged sequences than for both repeated sequences and novel sequences. Given that across repetitions both stimuli and nominal position information were matched, the only source of difference was the order of the stimuli. This suggests that the hippocampus is sensitive to the context (i.e., combination of item and position information) rather than specific items or positions themselves, consistent with the results found by Kalm and colleagues.
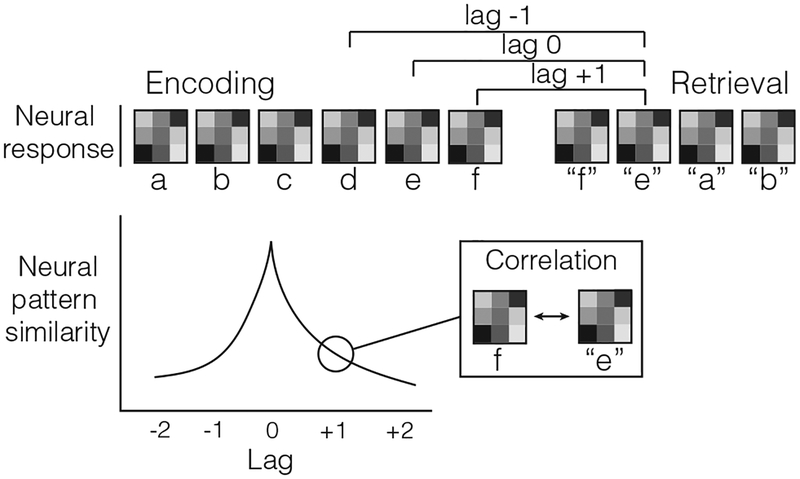
Thus far, we have shown evidence to suggest that the hippocampus is sensitive to contextual factors—the combination of stimuli and positions, rather than simply stimuli or positions alone. We now consider the hippocampal contribution to context reinstatement. Although the free recall and sequence learning results described above show that the hippocampus is sensitive to context, neural activity increases in the hippocampus may not directly reflect reinstatement. Instead, these signals could be directing reinstatement in other brain regions (Staresina, Henson, Kriegeskorte, & Alink, 2012; Ritchey, Wing, LaBar, & Cabeza, 2013). Comparison of hippocampal activity patterns during encoding with those during retrieval can thus address whether the hippocampus directly reinstates context (Howard, Viskontas, Shankar, & Fried, 2012; Yaffe et al., 2014; Yaffe, Shaikhouni, Arai, Inati, & Zaghloul, 2017). In a continuous recognition paradigm, Howard et al. (2012) assessed neural pattern similarity—the correlation in firing rate of medial temporal neurons—between the retrieval of a given target item and the encoding of its neighbors (Figure 2). The authors found evidence for contextual reinstatement: neural pattern similarity increased as the lag or distance between two items decreased. That is, neural pattern similarity was greatest when comparing retrieval of a target item to the encoding of its nearest neighbors (items ± 1 lag).
FIGURE 2.
Assessing contextual reinstatement. Neural responses, which can include firing rates across neurons, spectral power across electrodes, and/or BOLD activation across voxels, are measured during encoding and retrieval. Neural pattern similarity is a metric representing the correlations of neural patterns between encoding and retrieval trials. Pattern similarity is assessed at separate lags or differences in serial position between encoding and retrieval items. Retrieved-context theory predicts that neural pattern similarity between encoding and retrieval trials decreases as a function of absolute lag
Multivariate examination of the hippocampus during sequence learning has provided further evidence that the hippocampus specifically represents context (Hsieh, Gruber, Jenkins, & Ranganath, 2014). To dissociate contributions of item, position, and context information, Hsieh et al. (2014) ran an implicit sequence learning study while recording fMRI. Subjects learned fixed, random, and overlapping sequences. In fixed sequences, the same items were presented in the same positions across repetitions; for random sequences, the order was continually shuffled. For pairs of overlapping sequences, the first, fourth and fifth item differed across sequences, but the second and third items were matched.
Focusing on the random sequences, Hsieh et al. assessed neural pattern similarity of either the same items across repetitions or the same positions across repetitions. In each case, item or position information, respectively, is isolated and there is no influence of context by virtue of the fact that items and positions change on each presentation. Hsieh and colleagues found greater pattern similarity for the same items in the same positions in the nonrandom (fixed and overlapping) sequences than in either comparison for the random sequences. Together, these results suggest that the hippocampus represents the conjunction of item and position information.
The critical test of hippocampal context representations derives from the comparison of overlapping sequences. If the hippocampus specifically represents context, then pattern similarity should be greater for the same item in the same position in the same sequence, compared with the same item in the same position across sequences. To test this prediction, Hsieh et al. compared items within and across repetitions of the overlapping sequences. They found greater pattern similarity between repetitions of the same item in the same sequence (item B in Sequence 1, across repetitions), than between the same item across the overlapping sequences (item B in Sequences 1 and 2). As the only way to dissociate those instances of B is dependent on sequence membership, these results show that the hippocampus specifically represents contextual information.
Finally, there is also evidence that temporal context directly modulates stimulus representations (Schapiro, Kustner, & Turk-Browne, 2012; Schapiro, Rogers, Cordova, Turk-Browne, & Botvinick, 2013). Schapiro et al. (2012) had subjects view a series of fractals while performing an unrelated cover task. Unbeknownst to the subjects, the sequence of stimuli was composed of triplets that repeated throughout the experiment. By measuring the neural patterns associated with each stimulus both before and after the sequence task, the authors were able to assess whether the temporal context associated with a stimulus modifies its neural representation. Following repeated exposure, hippocampal pattern similarity between stimuli within a triplet increased. Thus, the representations of items that share a context become more similar following learning.
3 |. DISCUSSION
Evidence from varied recording modalities, data analytic approaches, and behavioral tasks all point to a linkage between hippocampal activity and serial-order memory. Yet, the precise mechanism underlying how the hippocampus supports order memory remains to be uncovered. This review suggests that many of these results could be synthesized under the framework of retrieved-context theory. This interim conclusion, however, awaits far more rigorous work aimed at understanding the exact neural representations in the hippocampus that support this type of coding, and the processes instantiated not only in the hippocampus, but also in cortical regions, that support serial-order memory more generally.
A critical question for retrieved-context theory is whether the context representation contains explicit position or timing information. The ability to determine the serial order of items that are not otherwise highly discriminable (e.g., the order of items within a triplet in Tubridy & Davachi, 2011 or the relative recency of items within a contextual boundary in DuBrow & Davachi, 2014) would seem to be dependent on the ability of context to contain an explicit representation of time. Recent discovery of hippocampal “time cells” suggests that the hippocampus may indeed directly represent time (Pastalkova, Itskov, Amarasingham, & Buzsáki, 2008; MacDonald, Lepage, Eden, & Eichenbaum, 2011; Salz et al., 2016). Time cells fire at specific delays relative to stimulus presentation and would appear to support the positional coding account, where elapsed time becomes associated with a stimulus presentation. However, electrophysiological data show that time cells do not merely respond to a temporal delay, but that they do so in a stimulus-specific manner (MacDonald et al., 2011). In a recent elaboration of retrieved-context theory, Howard and colleagues (Howard, Shankar, Aue, & Criss, 2015) propose a computational model that achieves a scale-invariant representation of internal history by allowing “cells” to have different delay constants—reflecting the behavior of actual time cells. The model is able to capture basic serial order behaviors across a range of tasks including judgments of recency, second order conditioning, and free recall. However, for both the model and the electrophysiological data, it is the conjunction of stimulus and time—that is, item and position information—that supports the pattern of observed results, a combination of information that is most consistent with the retrieved-context account. However, it is still an open question exactly how these cells may specifically support serial-order memory. Are differences in time cell firing patterns actually used in serial order judgments?
Likewise, questions remain regarding which hippocampal subfield(s) support serial-order memory performance. Temporal coding in animals has been observed in CA1 (Allen, Salz, McKenzie, & Fortin, 2016; MacDonald, Lepage, Eden, & Eichenbaum, 2011; Mankin et al., 2012), CA2 (Mankin et al., 2012), and CA3 (Salz et al., 2016). Work in humans has likewise found evidence for temporal coding across different hippo-campal subfields, where temporal contexts may be dissociated by CA1 (Dimsdale-Zucker, Ritchey, Ekstrom, Yonelinas, & Ranganath, 2018), CA2/CA3 (Copara et al., 2014) and both CA1 and CA2/CA3 (Kyle, Smuda, Hassan, & Ekstrom, 2015).
To adjudicate between these opposing pieces of data, future studies should use a comprehensive assessment of serial-order memory that combines a variety of the approaches used above. Specifically, both univariate and multivariate analytic techniques should be used to assess hippocampal subfield contributions to both the encoding and retrieval of serial order information. Univariate activity differences should track neural pattern similarity measures between items and across the encoding and retrieval of items.
Throughout this review, we have argued that serial-order memory is likely based on a hippocampally mediated process of associating items to a spatiotemporal context, such that reinstating that context enables retrieval of the items associated with that context. Such a mechanism, potentially based on the pattern completion processes of the hippocampus (Marr, 1971; Horner, Bisby, Bush, Lin, & Burgess, 2015), appears distinct from the pattern separating mechanisms of the hippocampus (Marr, 1971; McNaughton & Morris, 1987; O’Reilly & McClelland, 1994; Leutgeb, Leutgeb, Moser, & Moser, 2007; Yassa & Stark, 2011). Research on this process has shown that the hippocampus responds to highly similar or overlapping stimuli (Agster, Fortin, & Eichenbaum, 2002; Bakker, Kirwan, Miller, & Stark, 2008; Duncan, Curtis, & Davachi, 2009; Long, Lee, & Kuhl, 2016) and creates distinct representations of such stimuli, potentially in an effort to minimize interference (Hulbert & Norman, 2015; Favila, Chanales, & Kuhl, 2016; Chanales, Oza, Favila, & Kuhl, 2017).
Thus an intriguing question for future research concerns how the hippocampus can support serial-order memory, which relies on linking or associating representations, while concurrently engaging in the pattern separating processes that differentiate representations. The question of subfield involvement seems potentially critical here, as a dissociation (thus far, inconsistently observed) between subfields may enable the hippocampus to differentially support pattern separation and completion processes (Poppenk, Evensmoen, Moscovitch, & Nadel, 2013).
Although overwhelming evidence suggests that hippocampally mediated serial-order memory is driven by associations, there is another account of serial-order memory that does not rely on associations. Instead, strength theory proposes that order memory is supported by the strength of items (Hinrichs, 1970), where stronger items are judged to be more recent. Although strength may contribute partially to some of the neural effects described earlier, it clearly cannot be the sole explanation for the existing evidence on serial-order memory (Hintzman, 2003). It may be that subjects only rely on a unitary strength value when associative serial information is unavailable (Wells, 1974).
Serial information is only one of several dimensions along which memories can be organized. In addition to memories being organized by temporal factors, items can be organized by spatial associations (Burgess, Maguire, Spiers, & O’Keefe, 2001; Ekstrom et al., 2011; Miller et al., 2013) and by pre-experimental, longstanding semantic associations (Bousfield, Sedgewick, & Cohen, 1954; Long, Öztekin, & Badre, 2010; Polyn, Norman, & Kahana, 2009b; Morton et al., 2013). How does the hippocampus support these forms of organization? One possibility is that in its role as an associator, the hippocampus associates all contextual information, which can include spatial, semantic, and other nonserial forms of information, to an item. Specifically, other regions outside of the hippocampus, such as prefrontal cortex, may retrieve or select behaviorally relevant information that the hippocampus then associates to items (Long & Kahana, 2017), leading to other forms of nonserial organization during retrieval.
Although the focus of this review has been on how the hippocampus supports serial-order memory, a number of other regions likely work in concert with the hippocampus to support order memory. Evidence has shown that the parahippocampal cortex represents contextual information (Diana, Yonelinas, & Ranganath, 2012; Aminoff, Kveraga, & Bar, 2013). Likewise, lesion work has shown that individuals with frontal lobe lesions are impaired at serial-order memory (Mangels, Picton, & Craik, 2001, as well as nonserial organization memory Stuss et al., 1994; Alexander, Stuss, & Gillingham, 2009). Thus another direction of future research will be to investigate how other brain regions work with the hippocampus to support order memory.
Here we have synthesized neuro-imaging research from fMRI and iEEG studies investigating order memory, through the lens of cognitive theories of serial-order memory. Both the behavioral and neural evidence are consistent with the interpretation that the hippocampus supports both the formation and retrieval of item–context associations; however, exciting future questions about the exact mechanisms supporting these hippocampal associations await further study.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- Agster KL, Fortin NJ, & Eichenbaum H (2002). The hippocampus and disambiguation of overlapping sequences. Journal of Neuroscience, 22(13), 5760–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M, Stuss D, & Gillingham S (2009). Impaired list learning is not a general property of frontal lesions. Journal of Cognitive Neuroscience, 21(7), 1422–1434. [DOI] [PubMed] [Google Scholar]
- Allen TA, Salz DM, McKenzie S, & Fortin NJ (2016). Nonspatial sequence coding in ca1 neurons. The Journal of Neuroscience, 36(5), 1547–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, & Bar M (2013). The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences, 17(8), 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, & Bower GH (1972). Recognition and retrieval processes in free recall. Psychological Review, 79(2), 97–123. [Google Scholar]
- Bakker A, Kirwan CB, Miller M, & Stark CE (2008). Pattern separation in the human hippocampal ca3 and dentate gyrus. Science, 319(5870), 1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, & Plaut DC (2006). Short-term memory for serial order: A recurrent neural network model. Psychological Review, 113(2), 201–233. [DOI] [PubMed] [Google Scholar]
- Bousfield WA, Sedgewick CH, & Cohen BH (1954). Certain temporal characteristics of the recall of verbal associates. American Journal of Psychology, 67, 111–118. [PubMed] [Google Scholar]
- Brown GDA, Neath I, & Chater N (2007). A temporal ratio model of memory. Psychological Review, 114(3), 539–576. [DOI] [PubMed] [Google Scholar]
- Brown GDA, Preece T, & Hulme C (2000). Oscillator-based memory for serial order. Psychological Review, 107(1), 127–181. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, & O’Keefe J (2001). A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. NeuroImage, 14(2), 439–453. [DOI] [PubMed] [Google Scholar]
- Burke JF, Ramayya AG, & Kahana MJ (2015). Human intracranial high-frequency activity during memory processing: Neural oscillations or stochastic volatility? Current Opinion in Neurobiology, 31, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB (2015). Order-memory and association-memory. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie expérimentale, 69(3), 221–232. [DOI] [PubMed] [Google Scholar]
- Chanales AJ, Oza A, Favila SE, & Kuhl BA (2017). Overlap among spatial memories triggers repulsion of hippocampal representations. Current Biology, 27, 2307–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R (1965). Order error in immediate recall of sequences. Journal of Verbal Learning and Verbal Behavior, 4(3), 161–169. [Google Scholar]
- Copara MS, Hassan AS, Kyle CT, Libby LA, Ranganath C, & Ekstrom AD (2014). Complementary roles of human hippocampal subregions during retrieval of spatiotemporal context. The Journal of Neuroscience, 34(20), 6834–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, & DuBrow S (2015). How the hippocampus preserves order: The role of prediction and context. Trends in Cognitive Sciences, 19(2), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, & Wagner AD (2003). Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences of the United States of America, 100(4), 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2012). Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia, 50, 3062–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale-Zucker HR, Ritchey M, Ekstrom AD, Yonelinas AP, & Ranganath C (2018). Ca1 and ca3 differentially support spontaneous retrieval of episodic contexts within human hippocampal subfields. Nature Communications, 9(1), 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S, & Davachi L (2013). The influence of contextual boundaries on memory for the sequential order of events. Journal of Experimental Psychology: General, 142(4), 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S, & Davachi L (2014). Temporal memory is shaped by encoding stability and intervening item reactivation. The Journal of Neuroscience, 34(42), 13998–14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Curtis C, & Davachi L (2009). Distinct memory signatures in the hippocampus: Intentional states distinguish match and mismatch enhancement signals. The Journal of Neuroscience, 29(1), 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbinghaus H (1885/1913). On memory: A contribution to experimental psychology. New York: Teachers College, Columbia University. [Google Scholar]
- Eichenbaum H (2017). Time (and space) in the hippocampus. Current Opinion in Behavioral Sciences, 17, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, & Bookheimer SY (2007). Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learning & Memory, 14(10), 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang W, & Yonelinas AP (2011). Dissociable networks involved in spatial and temporal order source retrieval. NeuroImage, 56(3), 1803–1813. [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, & Davachi L (2014). Similarity breeds proximity: Pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron, 81(5), 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, & Eichenbaum H (2010). Distinct roles for dorsal ca3 and ca1 in memory for sequential nonspatial events. Learning & Memory, 17(1), 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favila SE, Chanales AJ, & Kuhl BA (2016). Experience-dependent hippocampal pattern differentiation prevents interference during subsequent learning. Nature Communications, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, & Eichenbaum HB (2002). Critical role of the hippocampus in memory for sequences of events. Nature, 5(5), 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin DR, & Mewhort D (2015). Memory as a hologram: An analysis of learning and recall. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Expérimentale, 69(1), 115. [DOI] [PubMed] [Google Scholar]
- Friedman WJ (1993). Memory for the time of past events. Psychological Bulletin, 113(1), 44. [Google Scholar]
- Henson R (1998). Short-term memory for serial order: The start-end model. Cognitive Psychology, 36, 73–137. [DOI] [PubMed] [Google Scholar]
- Hinrichs JV (1970). A two-process memory-strength theory for judgment of recency. Psychological Review, 77(3), 223. [Google Scholar]
- Hintzman DL (2003). Judgments of recency and their relation to recognition memory. Memory & Cognition, 31(1), 26–34. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Bisby JA, Bush D, Lin W-J, & Burgess N (2015). Evidence for holistic episodic recollection via hippocampal pattern completion. Nature communications, 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, & Kahana MJ (1999). Contextual variability and serial position effects in free recall. Journal of Experimental Psychology: Learning, Memory, and Cognition, 25(4), 923–941. [DOI] [PubMed] [Google Scholar]
- Howard MW, & Kahana MJ (2002). A distributed representation of temporal context. Journal of Mathematical Psychology, 46(3), 269–299. [Google Scholar]
- Howard MW, Shankar KH, Aue WR, & Criss AH (2015). A distributed representation of internal time. Psychological Review, 122(1), 24–53. [DOI] [PubMed] [Google Scholar]
- Howard MW, Viskontas IV, Shankar KH, & Fried I (2012). Ensembles of human MTL neurons “jump back in time” in response to a repeated stimulus. Hippocampus, 22, 1833–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L-T, Gruber M, Jenkins L, & Ranganath C (2014). Hippocampal activity patterns carry information about objects in temporal cortex. Neuron, 81, 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert J, & Norman K (2015). Neural differentiation tracks improved recall of competing memories following interleaved study and retrieval practice. Cerebral Cortex, 25(10), 3994–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, & Ranganath C (2016). Distinct neural mechanisms for remembering when an event occurred. Hippocampus, 26, 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalm K, Davis MH, & Norris D (2013). Individual sequence representations in the medial temporal lobe. Journal of Cognitive Neuroscience, 25(7), 1111–1121. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, & Barua LA (2002). The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience, 116(2), 286–290. [DOI] [PubMed] [Google Scholar]
- Konishi S, Uchida I, Machida T, Shirouzu I, & Miyashita Y (2002). Neural correlates of recency judgment. The Journal of Neuroscience, 22(21), 9549–9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel JE, Morton NW, & Polyn SM (2015). Neural activity in the medial temporal lobe reveals the fidelity of mental time travel. The Journal of Neuroscience, 35(7), 2914–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, & Maguire EA (2005). The human hippocampus: Cognitive maps or relational memory? The Journal of Neuroscience, 25(31), 7254–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, & Maguire EA (2006a). The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron, 49(4), 617–629. [DOI] [PubMed] [Google Scholar]
- Kumaran D, & Maguire EA (2006b). An unexpected sequence of events: Mismatch detection in the human hippocampus. PLoS Biol, 4(12), e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle CT, Smuda DN, Hassan AS, & Ekstrom AD (2015). Roles of human hippocampal subfields in retrieval of spatial and temporal context. Behavioural Brain Research, 278, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn H, Steffenach H-A, van Strien NM, Veltman DJ, Witter MP, & Håberg AK (2009). A specific role of the human hippocampus in recall of temporal sequences. Journal of Neuroscience, 29(11), 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb J, Leutgeb S, Moser M, & Moser E (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315(5814), 961. [DOI] [PubMed] [Google Scholar]
- Lewandowsky S, & Murdock BB (1989). Memory for serial order. Psychological Review, 96(1), 25–57. [Google Scholar]
- Lohnas LJ, Polyn SM, & Kahana MJ (2015). Expanding the scope of memory search: Modeling intralist and interlist effects in free recall. Psychological Review, 122(2), 337. [DOI] [PubMed] [Google Scholar]
- Long NM, & Kahana MJ (2015). Successful memory formation is driven by contextual encoding in the core memory network. NeuroImage, 119, 332–337. [DOI] [PubMed] [Google Scholar]
- Long NM, & Kahana MJ (2017). Modulation of task demands suggests that semantic processing interferes with the formation of episodic associations. Journal of Experimental Psychology: Learning, Memory, and Cognition, 43(2), 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, Lee H, & Kuhl BA (2016). Hippocampal mismatch signals are modulated by the strength of neural predictions and their similarity to outcomes. Journal of Neuroscience, 36(50), 12677–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, Öztekin I, & Badre D (2010). Seperable prefrontal cortex contributions to free recall. The Journal of Neuroscience, 30(33), 10967–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, Sperling MR, Worrell GA, Davis KA, Gross RE, & Lega BC. (2017). Contextually mediated spontaneous retrieval is specific to the hippocampus. Current Biology, 27(7), 1074–1079.others [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald C, Lepage K, Eden U, & Eichenbaum H (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron, 71(4), 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels J, Picton T, & Craik FIM (2001). Attention and successful episodic encoding: An event-related potential study. Cognitive Brain Research, 11, 77–95. [DOI] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, & Leutgeb JK (2012). Neuronal code for extended time in the hippo-campus. Proceedings of the National Academy of Sciences of the United States of America, 109(47), 19462–19467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, & Eichenbaum H (2007). Gradual changes in hippocampal activity support remembering the order of events. Neuron, 56(3), 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1971). Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society B: Biological Sciences, 262(841), 23–81. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, & Morris RG (1987). Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences, 10, 408–415. [Google Scholar]
- Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, … Schulze-Bonhage A (2013). Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science, 342(6162), 1111–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Zanos S, Fetz EE, den Nijs M, & Ojemann J (2009). Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. The Journal of Neuroscience, 29(10), 3132–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C, Poirier M, Fortin C, & Hulme C (2006). Word frequency and the mixed-list paradox in immediate and delayed serial recall. Psycho-nomic Bulletin & Review, 13(4), 724–729. [DOI] [PubMed] [Google Scholar]
- Morton NW, Kahana MJ, Rosenberg EA, Sperling MR, Sharan AD, & Polyn SM (2013). Category-specific neural oscillations predict recall organization during memory search. Cerebral Cortex, 23(10), 2407–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, & Malach R (2005). Coupling between neuronal firing, field potentials, and fMRI in human auditory cortex. Science, 309(5736), 951–954. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, & McClelland JL (1994). Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus, 4(6), 661–682. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, & Buzsáki G (2008). Internally generated cell assembly sequences in the rat hippocampus. Science, 321(5894), 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, & Kahana MJ (2008). Memory search and the neural representation of context. Trends in Cognitive Sciences, 12, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Norman KA, & Kahana MJ (2009a). A context maintenance and retrieval model of organizational processes in free recall. Psychological Review, 116, 129–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Norman KA, & Kahana MJ (2009b). Task context and organization in free recall. Neuropsychologia, 47, 2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, & Nadel L (2013). Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, & D’Esposito M (2004). Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia, 42(1), 2–13. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, LaBar KS, & Cabeza R (2013). Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cerebral Cortex, 23(12), 2818–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Brown TI, & Stern CE (2009). The retrieval of learned sequences engages the hippocampus: Evidence from fmri. Hippocampus, 19(9), 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz DM, Tiganj Z, Khasnabish S, Kohley A, Sheehan D, Howard MW, & Eichenbaum H (2016). Time cells in hippocampal area ca3. Journal of Neuroscience, 36(28), 7476–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Kustner LV, & Turk-Browne NB (2012). Shaping of object representations in the human medial temporal lobe based on temporal regularities. Current Biology, 22(17), 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Rogers TT, Cordova NI, Turk-Browne NB, & Botvinick MM (2013). Neural representations of events arise from temporal community structure. Nature Neuroscience, 16(4), 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, & Stern CE (2003). An fmri study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron, 37(6), 1013–1025. [DOI] [PubMed] [Google Scholar]
- Shankar KH, & Howard MW (2012). A scale-invariant internal representation of time. Neural Computation, 24, 134–193. [DOI] [PubMed] [Google Scholar]
- Solway A, Murdock BB, & Kahana MJ (2012). Positional and temporal clustering in serial order memory. Memory & Cognition, 40(2), 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Henson RN, Kriegeskorte N, & Alink A (2012). Episodic reinstatement in the medial temporal lobe. The Journal of Neuro-science, 32(50), 18150–18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Palumbo CL, Buckle L, Sayer L, & Pogue J (1994). Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology, 8, 355–355. [Google Scholar]
- Suzuki WA (2007). Making new memories. Annals of the New York Academy of Sciences, 1097(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Tubridy S, & Davachi L (2011). Medial temporal lobe contributions to episodic sequence encoding. Cerebral Cortex, 21(2), 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, & Diana RA (2016). Temporal context processing within hippocampal subfields. NeuroImage, 134, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JE (1974). Strength theory and judgments of recency and frequency. Journal of Verbal Learning and Verbal Behavior, 13(4), 378–392. [Google Scholar]
- Yaffe RB, Kerr MS, Damera S, Sarma SV, Inati SK, & Zaghloul KA (2014). Reinstatement of distributed cortical oscillations occurs with precise spatiotemporal dynamics during successful memory retrieval. Proceedings of the National Academy of Sciences of the United States of America, 111(52), 18727–18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe RB, Shaikhouni A, Arai J, Inati SK, & Zaghloul KA (2017). Cued memory retrieval exhibits reinstatement of high gamma power on a faster timescale in the left temporal lobe and prefrontal cortex. Journal of Neuroscience, 37(17), 4472–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, & Stark CE (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34(10), 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema DB, & Trask FP (1963). Recall as a search process. Journal of Verbal Learning and Verbal Behavior, 2, 65–74. [Google Scholar]
- Young RK (1962). Tests of three hypotheses about the effective stimulus in serial learning. Journal of Experimental Psychology, 63(3), 307–313. [DOI] [PubMed] [Google Scholar]