Figure 2. Examples of modulating the Ub-ligase/deubiquitinase network in cancer.
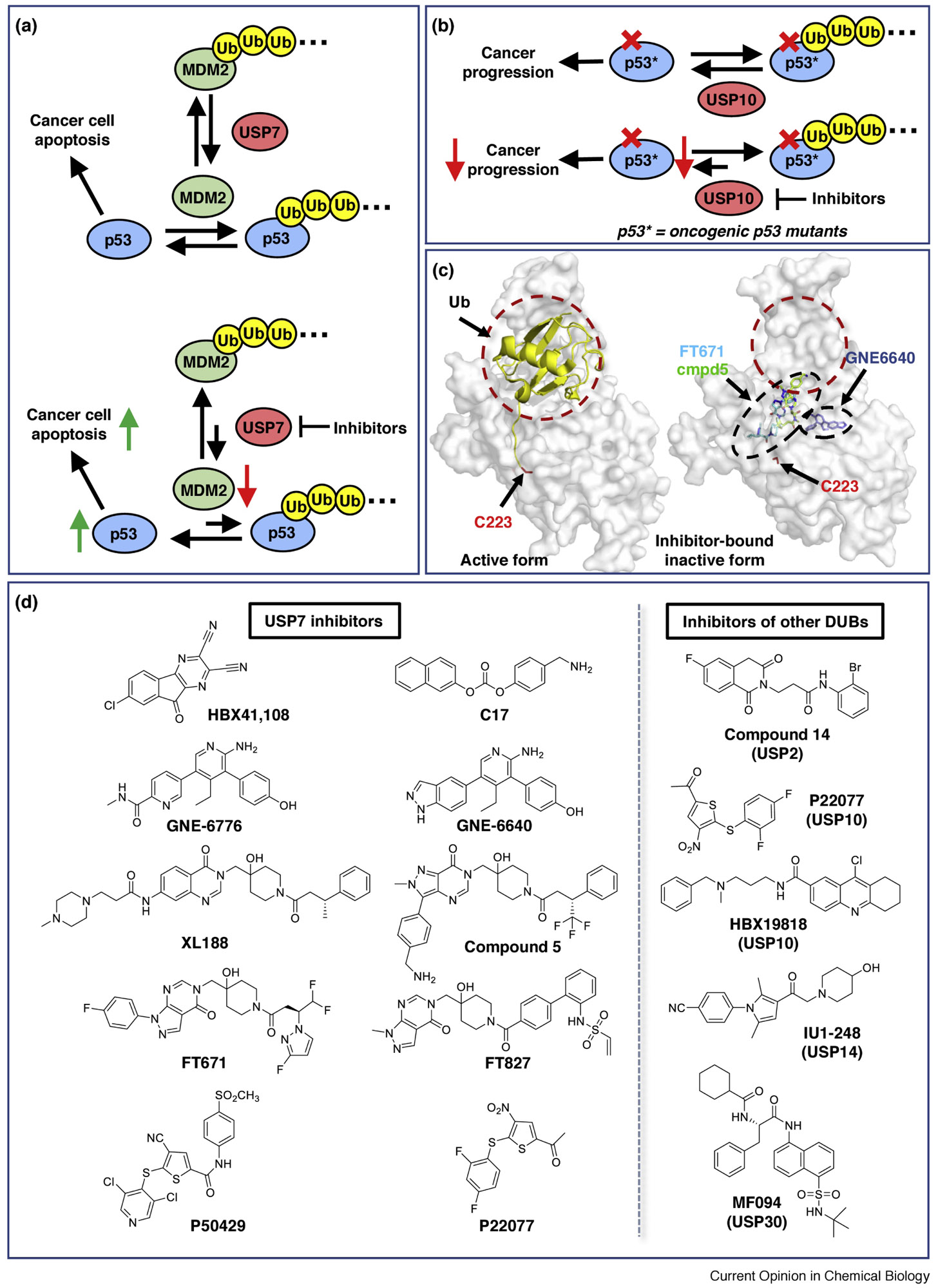
(a) USP7 functions as a positive regulator of tumor suppressor p53 by deubiquitylating MDM2 (top). Inhibition of USP7 should destabilize MDM2, causing an upregulation of p53 and hence tumor suppression (bottom). (b) USP10 deubiquitylates wild-type and oncogenic mutant p53 (top). Inhibition of USP10 increases the turnover of both wild-type and mutant p53 and prevents cancer progression in p53-mutant-driven cancer cells (bottom). (c) Illustration of allosteric small molecules binding to the USP7 catalytic domain. Ubiquitin (yellow cartoon) binds to USP7 (grey) at a broad, dynamic interface (red circle) with its C-terminus inserted into a catalytic pocket, and induces USP7 to adopt an active conformation (left, PDB 1NBF). Representative small molecules (sticks) overlaid on an inactive form of USP7 (right, PDB 5N9R). GNE6640 (blue, PDB 5UQV), FT671 (cyan, PDB 5NGE), and compound 5 (green, 5N9T) bind at distal regions from the Ub-binding interface, forcing USP7 to adopt an inactive conformation that sterically occludes ubiquitin binding. The catalytic Cys223 is highlighted. (d) Left panel: recent examples of USP7 inhibitors that are discussed in this review. Right panel: examples of other DUB inhibitors (right, more details see [17]).
