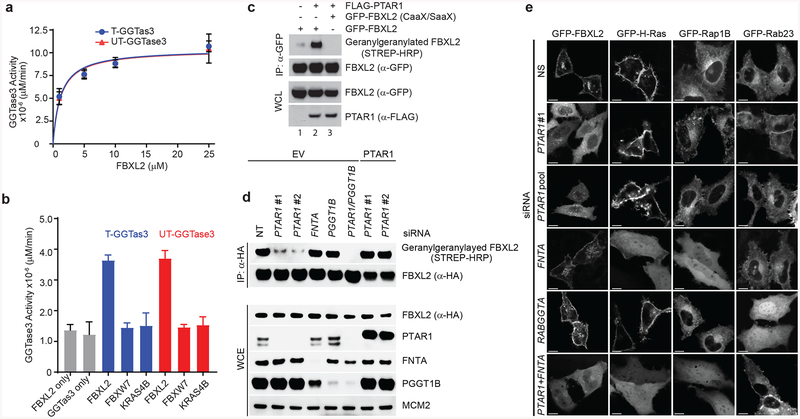
Figure 2. GGTase3 geranylgeranylates FBXL2 and is required for its localization to cellular membranes.
(a) Recombinant GGtase3 geranylgeranylates purified FBXL2. Indicated amounts of purified FBXL2 were incubated with 100 ng of purified GGTase3 (either tagged [T] or untagged [UT] versions) to carry out in vitro geranylgeranylation assay using saturating concentrations of tritiated [H3]-GGPP as described in methods. Each data point represents mean+/− SD of three biological replicates. Michaelis-Menten kinetics was used to generate an apparent Km value of 1.2μM using Prism Graphpad software.
(b) In vitro geranylgeranylation assay was carried out and measured as in (a) using 10 μM of purified FBXL2, FBXW7, or K-RAS4B and 100 ng of purified GGTase3. Bar graphs represent mean +/− SD from three biological replicates. Source data for panels a and b are available with the paper online.
(c) RPE1-HTERT cells were cotransfected with the indicated plasmids and processed for the detection of geranylgeranylated FBXL2 using a “Click-IT” assay, as described in methods. The experiment was repeated three times. Representative result is shown. Uncropped blot/gel images are shown in Supplementary Data Set 1.
(d) HeLa cells were transfected with the indicated siRNA oligos and cDNAs. Twenty-four hours post-transfection cells were incubated with geranylgeranyl-azide for 16 hours, harvested, lysed, and azide selective ligation reaction with sDIBO-Biotin was performed for one hour to label geranylgeranylated proteins via copper-free “Click-IT” reaction. After immunoprecipitation with an anti-HA antibody, immunoblots were carried out. The experiment was repeated four times. Representative result is shown. Uncropped blot/gel images are shown in Supplementary Data Set 1.
(e) HeLa cells were transfected first with the indicated siRNA oligos and then with the indicated GFP-tagged proteins. Live cell confocal imaging was carried out as described in methods. Images show representative frames of three independent experiments. Bar size: 10 μm.