Fig. 2.
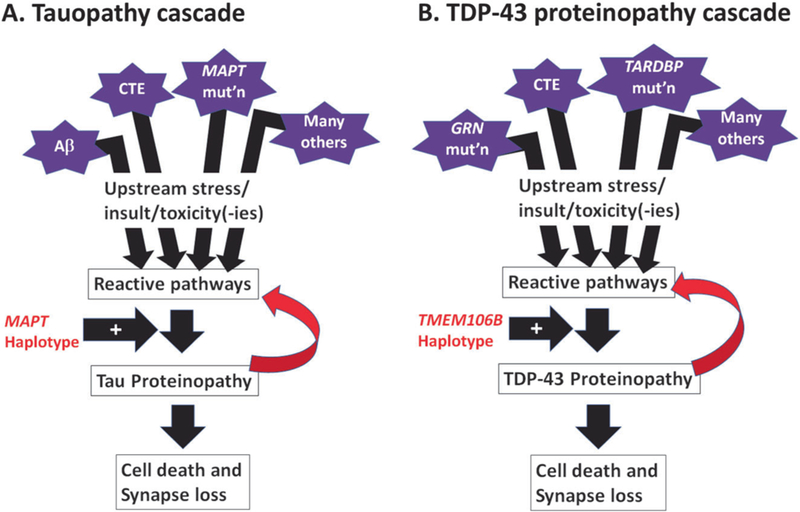
Schematic cartoons depict the overlapping elements of the pathological cascades that are seen in Tau (a) and TDP-43 (b) proteinopathies. Multiple factors can contribute to Tau or TDP-43 proteinopathies, or both (see Table 1). For Tau and TDP-43 proteins, reactive pathways—perhaps related to oxidative stress, phosphorylation, autophagy, proteolysis, inflammation, and/or other biochemical changes—appear to have an influence on the proteins’ structural properties, making the polypeptides more likely to misfold and to generate both toxicity and loss of normal function. Whereas the MAPT haplotype appears to be an important genetic risk factor in multiple different Tau proteinopathies, the TMEM106B haplotype (signaled by the rs1990662 risk variant) is associated with increased risk for multiple TDP-43 proteinopathies. Both Tau and TDP-43 proteinopathies also appear to be “transmissible” in animal models (see ref. [10]), meaning that their presence in the brain can—even without another “upstream factor”—potentiate additional “downstream” proteinopathy, contributing to cell death and synapse loss
