Fig. 4.
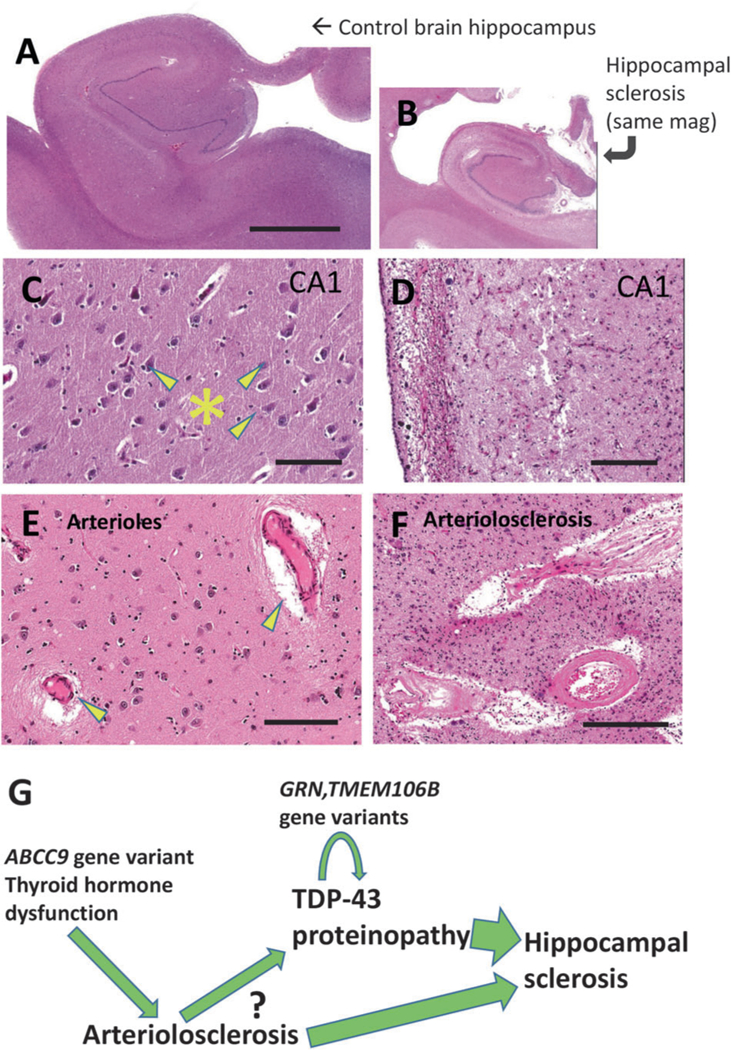
TDP-43 proteinopathy with hippocampal sclerosis (HS) pathology and arteriolosclerosis pathology provide the basis for a hypothetical pathologic cascade in aged human brains. Shown are aged control brain (a, c, e) and HS brain (b, d, f) for comparison’s sake, stained using H&E. This HS brain is the same one that is depicted in Fig. 3. Note that the photomicrographs in (a) and (b) are at the same magnification, indicating the amount of atrophy in the hippocampus shown in (b). Portions of the hippocampal CA1 sector are shown for comparison in panels c, d. Note that the control brain has large pyramidal neurons (arrowheads) and dense, intact eosinophilic neuropil (*). By contrast, the CA1 sector in the brain with HS pathology shows astrocytosis, dropout of neurons, and neuropil that is looser and rarefied. Brain arterioles that have histopathologic features within normal limits in aged brains (arrowheads in panel e) can be contrasted with arteriolosclerosis pathology (f) where the arteriolar walls are thickened and dysmorphic with eosinophilic material in the vessel wall that may impair cerebral blood flow. A hypothetical sequence, influenced by various factors, is shown (g) that incorporates findings from multiple prior studies [48, 92, 94, 144–148]. Upstream genetic risk factors may contribute to brain arteriolosclerosis in a manner that induces chronic stress in the brain, potentiating TDP-43 proteinopathy that also is influenced by additional genetic risk factors (e.g., TMEM106B, GRN). The question-mark conveys that the detailed mechanisms are as yet mostly unknown. The combination of ‘upstream’ stresses and TDP-43 proteinopathy may contribute to the cell loss and gliosis that manifests as HS pathology and contributes to the dementia syndrome. Scale bars = 6 mm (a, b), 120 μm (c, d), and 200 μm (f, g)
