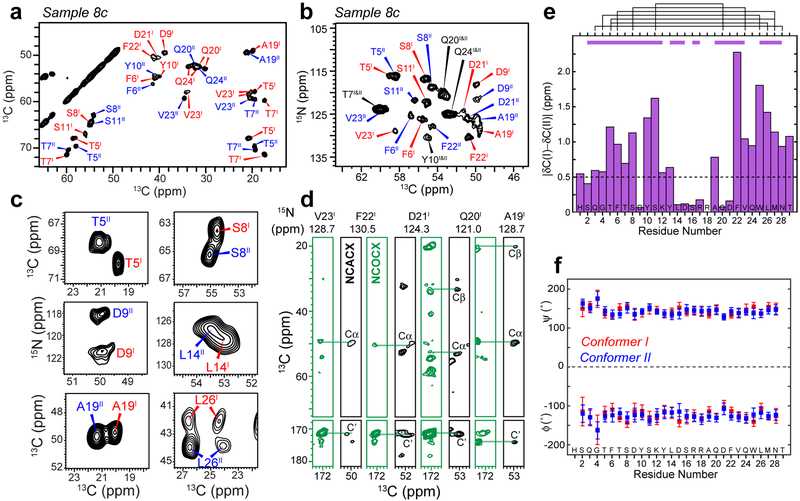
Figure 2.
Glucagon fibrils contain two distinct β-strand conformations. a, Representative 2D 13C-13C correlation spectrum with 30 ms 13C spin diffusion (SD), showing well resolved chemical shifts of the glucagon fibrils. b, Representative 2D 15N-13Cα correlation spectrum. c, Selected 2D 13C-13C and 15N-13C spectra reveal peak doubling, indicating the coexistence of two molecular conformations. d, Strips of 3D NCACX (black) and NCOCX (green) correlation spectra, illustrating sequential resonance assignment. e, Absolute chemical shift differences between conformers I and II. Purple bars above the diagram indicate segments for which conformers I and II are unambiguously assigned from the sequential cross peaks, whereas black lines indicate connectivities between residues that are far away in the amino acid sequence, which help to distinguish conformer I and conformer II. f, Backbone (ϕ, ψ) torsion angles obtained from the experimental 13C and 15N chemical shifts. Both conformers correspond to a continuous β-strand.