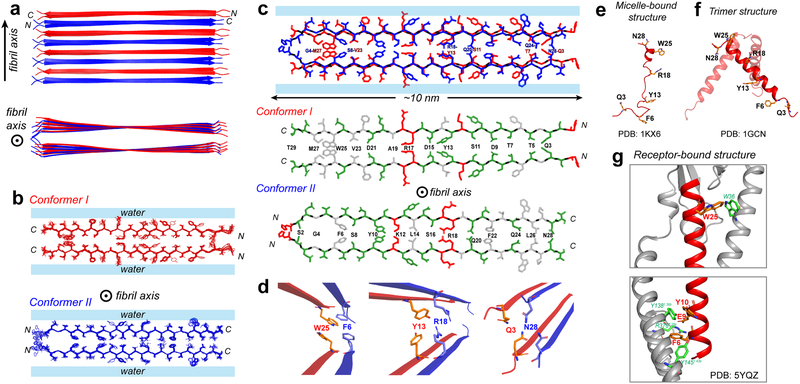
Figure 4.
Atomic structure of the low-pH glucagon fibril. a, Top and side view of the fibril. Conformer I (red) and conformer II (blue) alternate and hydrogen-bond in antiparallel. Each conformer forms a parallel homodimer as the basic repeat unit in the cross section normal to the fibril axis. b, Ensemble of 10 lowest-energy structures viewed down the fibril axis. For clarity, conformers I and II are shown separately. c, Sidechain packing in the dimer-of-dimer subunit of glucagon fibrils. Each conformer self-associates in parallel, exhibiting C2 symmetry in the cross section normal to the fibril axis. Conformer I packs odd-numbered residues at the steric zipper interface whereas conformer II packs even-numbered residues at the steric zipper interface. Hydrophobic sidechains are colored white, polar residues are green and positively charged residues are red. d, Zoomed-in views of F6–W25, Y13-R18 and Q3–N28 sidechain packing, which stabilize the fibril structure. This antiparallel fibril structure contrasts with the glucagon structures in solution and as bound to its receptor. e, The micelle-bound glucagon is mostly disordered (PDB 1KX6)43,44. f, The crystal structure of glucagon (PDB 1GCN) is predominantly an α-helical trimer, which may be the precursor to the β-sheet fibril. g, When bound to its G-protein coupled receptor in the lipid membrane, glucagon is fully α-helical45 (PDB5YQZ) and has many stabilizing interactions with the receptor.