Extended Data Fig.1. Circulating antibodies are incapable of entering vaginal mucosal lumen in the absence of minor abrasion.
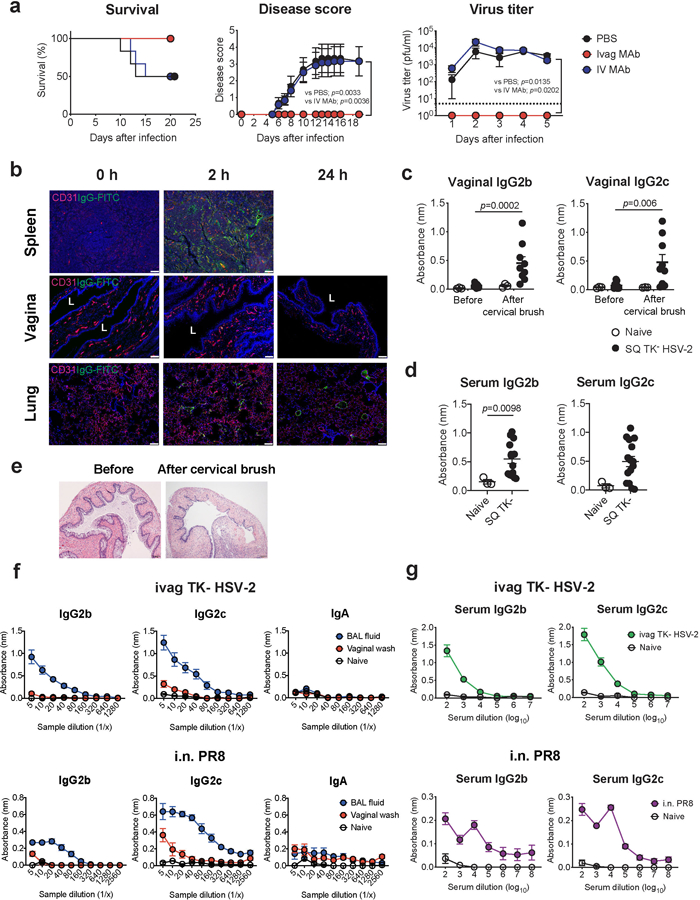
a, C57BL/6 mice treated intravaginally or intravenously with HSV gD-specific MAb were infected with intravaginal WT HSV-2. Survival, disease severity, and virus titer in vaginal wash were analyzed (n=6). Dashed line indicates limit of detection. b, C57BL/6 mice treated with depo-provera were injected intravenously with FITC-conjugated mouse IgG antibody. Frozen sections of vagina, spleen and lung were stained with anti-CD31 (red), anti-FITC (green) antibodies, and DAPI (blue). L indicates vaginal lumen. Scale bars indicate 100 μm. c-e, C57BL/6 mice were immunized subcutaneously with TK− HSV-2. Five weeks later, the vaginal tract of immunized mice with depo treatment was brushed with cervical brush to make a breach or minor abrasions of the epithelial barrier of the vaginal mucosa. HSV-specific antibodies in vaginal wash before and after cervical brush (c, naïve; n=3, SQ TK− HSV-2; n=9) and in serum (d, naïve; n=3, SQ TK− HSV-2; n=15) were measured by ELISA. Sample dilution for ELISA was 1:2 (vaginal wash) or 1:103 (serum). e, Hematoxylin and eosin (H&E) stain of the vagina before and after breach was performed in mice with depo treatment. f-g, C57BL/6 mice were immunized intravaginally with TK− HSV-2 or intranasally with influenza A/PR8 (H1N1) virus. Five weeks later, virus-specific antibodies in bronchoalveolar lavage (BAL) fluid, vaginal wash (f, naïve; n=4, ivag TK− HSV-2; n=6, i.n. PR8; n=4), and blood (g, naïve; n=4, ivag TK− HSV-2; n=13, i.n. PR8; n=8) were measured by ELISA. Data are mean ± SEM. Data are representative of two independent experiments (a,b,e) or are pooled from two independent experiments (c-d,f-g). Statistical significance was analyzed by two-way ANOVA (a; disease score, virus titer), log- rank (Mantel-Cox) test (a; survival) or two-tailed Mann-Whitney U test (c,d).
