Figure 1.
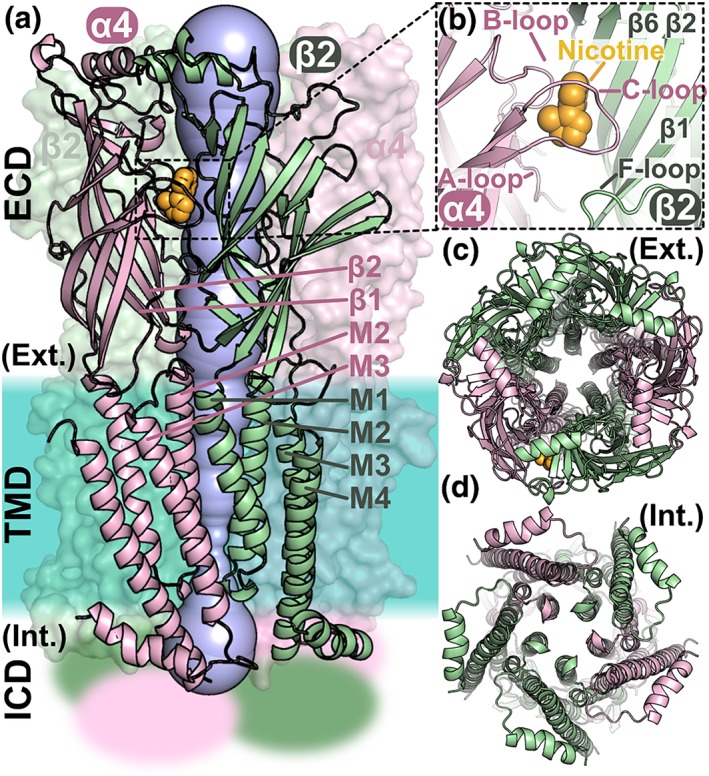
Structure of the heteropentameric ligand‐gated ion channel α4β2 nAChR. (a) Domain and subunit organisation of the α4β2 nAChR and visualisation of the pore. The α4 and β2 subunits are coloured pink and green, respectively. The α4 and β2 subunits on the front shown are in cartoon representation and the other three subunits in surface representation. The nAChR is composed of three domains: an extracellular domain (ECD), a transmembrane domain (TMD), and an intracellular domain (ICD), the structure of which is unknown and is represented as fuzzy areas. The crystal structure of the α4β2 nAChR (PDB identifier 5kxi) was used to draw this figure. A nicotine molecule, coloured in orange, is bound in the orthosteric binding pocket. The pore of the channel is shown in blue, and the approximate location of the membrane region is shown as a turquoise area. (b) Orthosteric binding site occupied by a nicotine molecule. The A‐, B‐, and C‐loops of the α4 subunits are labelled as is the F‐loop and several β‐strands from the β4 subunit. (c) View through the ECD of the central pore from the extracellular (Ext.) side. (d) View through the TMD of the central pore from the intracellular (Int.) side. The M2 helices of the TMD are lining the pore of the channel
