Figure 3.
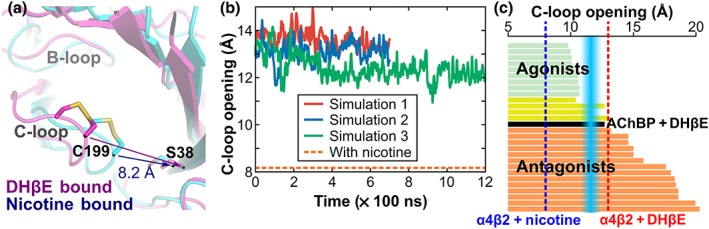
Conformation of C‐loop of the α4β2 nAChR in complex with DHβE and comparison with ACh binding proteins (AChBPs) in complex with agonists and antagonists. (a) Superimposition of a molecular model of the complex between α4β2 nAChR and DHβE (magenta) and of the X‐ray crystallographic structure of the human α4β2 nicotinic receptor interacting with nicotine (blue). The distance used to characterise the C‐loop opening in the α4β2 nAChR was measured between the Cα of Cys199 of the α4 subunit and the Cα of Ser38 of the β2 subunit. (b) Distance characteristic of the C‐loop “opening” (averaged between the two binding sites) during the three MD simulations of α4β2 nAChR/DHβE complex. The orange dashed line represents the C‐loop opening in the nicotine/α4β2 nAChR complex (PDB identifier 5kxi). (c) C‐loop opening distance in crystal structures of AChBP in complex with agonists (green), antagonists (orange), or compounds with dual activity (yellow). The blue zone represents the approximate boundary between agonists and antagonists. The red dashed line shows the average C‐loop distance measured during the MD simulations of the α4β2 nAChR/DHβE system. The blue dashed lines represent the C‐loop opening distance in the crystal structure of the α4β2 nAChR/nicotine complex. The AChBP structures that were analysed in panel (c) are listed in Table S3. The evolution of the C‐loop opening distance of each binding site for the three simulations is shown in Figure S2
