Figure 7.
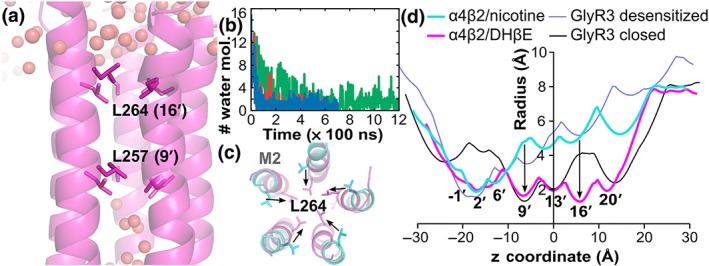
Pore in the TMD of the α4β2 nAChR when in complex with DHβE. (a) Distribution of water molecules (spheres) in the TMD channel of the α4β2 nAChR bound with DHβE in the final frame of one of the simulations. (b) Number of water molecules passing the gate L257 over the time of the three simulations. Simulations 1, 2, and 3 are in red, blue, and green, respectively. (c) Overlay of the structures of the M2 helices forming the pore in the TMD in the final frame of Simulation 1 of the α4β2/DHβE complex (magenta) and of the crystal structure of the desensitised state (PDB identifier 5kxi, cyan) as viewed from the extracellular side of the membrane. (d) Comparison of the TMD channel radius profiles of the α4β2 nAChR and GlyR3 in the closed/resting and desensitised states. The radius profiles were computed using Hole. Residues L257 (9′) and L264 (16′) create the main constrictions in the pore radius profiles, and these gate residues are shown in stick representation in panels (a) and (c)
