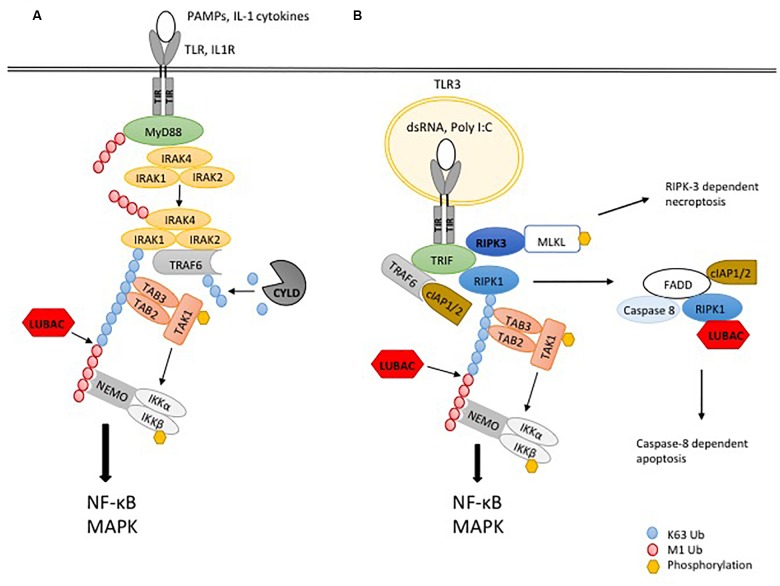
FIGURE 2.
Ubiquitylation in TLR signaling. (A) The recognition of PAMPs or IL-1α/β by the respective TLR or IL-1R leads to binding of the adaptor protein MyD88, which interacts with the TIR domain of the receptor, leading to recruitment of the kinases IRAK4, IRAK1 and IRAK2. Dissociation of IRAK1 and IRAK2 and their interaction with the ubiquitin ligase TRAF6 initiates the formation of K63-linked poly-ubiquitin chains, serving as a platform for recruitment of TAB2 and TAB3 to activate the kinase TAK1 and in turn MAPK signaling. In addition, the ubiquitin ligase LUBAC extends K63-linked poly-ubiquitin chains with M1-linked poly-ubiquitin chains, resulting in recruitment and activation of the IKK complex through its adaptor NEMO. Phosphorylation of IKKβ by TAK1 leads to activation of NF-κB and pro-inflammatory signaling. The DUB CYLD counteracts ubiquitylation by TRAF6. (B) TLR3 signaling works independently of MyD88 but requires the adaptor TRIF. After binding of dsRNA or Poly I:C to the receptor, the kinase RIPK1 is recruited. The ubiquitin ligases TRAF6 and cIAP1/2 generate K63-linked poly-ubiquitin chains to activate TAK1 and downstream MAPK. To induce the activation of IKKs and NF-κB, LUBAC is recruited to the complex to generate M1-linked poly-ubiquitin chains. If K63- and/or M1-ubiquitylation are blocked, apoptosis is induced by a complex including FADD, caspase-8 and RIPK1.