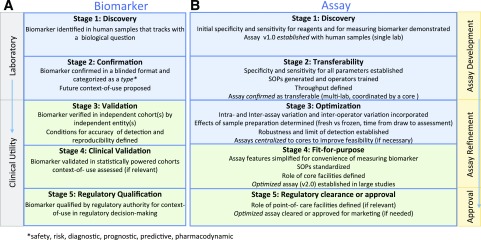
Figure 3.
Proposed stages of development for T1D biomarkers and assays (consensus view of the authors). Biomarkers and their associated assays have parallel and independent lines of development, ideally converging at the stage of biomarker validation using fit-for-purpose assays for reliable use by the scientific community. A: A biomarker must successfully pass through stage 3 to be considered validated for research purposes. If a biomarker is a candidate for regulatory decision-making, subsequent stages of development (stages 4–5) must be completed. B: All assays should ideally achieve fit-for-purpose status (stage 4) for widespread use to measure a validated biomarker. In specific instances, where an assay has achieved approval for marketing purposes, it must be cleared by regulatory bodies (stage 5). SOPs, standard operating procedures.