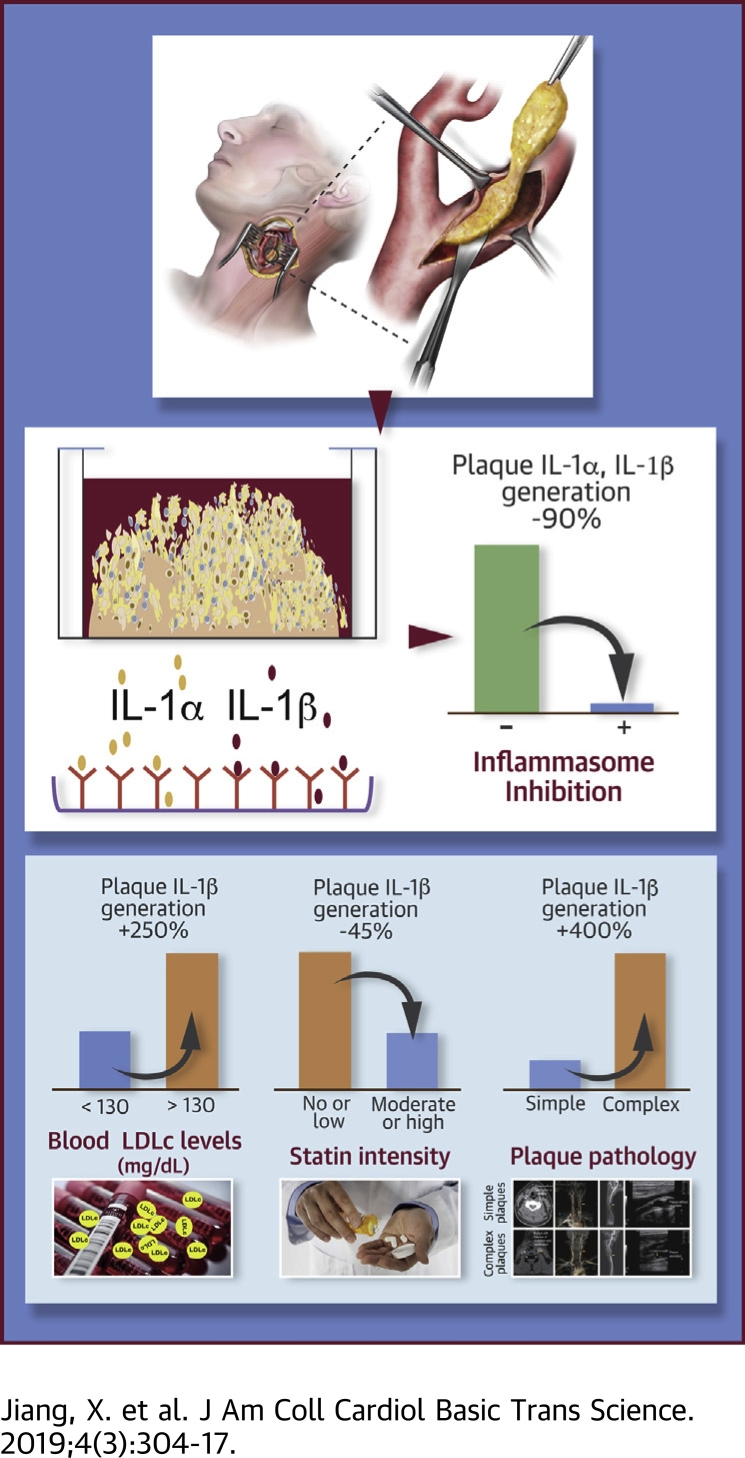
Visual Abstract
Key Words: atherosclerosis, hypercholesterolemia, inflammasome, inflammation, interleukin-1
Abbreviations and Acronyms: ASC, apoptosis-associated speck-like protein containing a CARD; ATP, adenosine 5′-triphosphate disodium salt hydrate; BiKE, Biobank of Karolinska Carotid Endarterectomies; CT, Computerized tomographic scanning; IL, interleukin; LDL, low-density lipoprotein; LPS, lipopolysaccharide; mRNA, messenger ribonucleic acid; NLRC, nucleotide-binding oligomerization domain, leucine-rich repeat and CARD domain–containing protein; NLRP, nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing protein; PBS, phosphate-buffered saline
Highlights
-
•
Genetic and functional evidence suggests that there are additional inflammasome pathways, besides NLRP3, that contribute to IL-1 generation in human atherosclerotic plaques.
-
•
Plaque generation of mature IL-1β is accompanied by secretion of similar levels of IL-1α, through a mechanism controlled by NLRP3 and caspase-1.
-
•
Plaque IL-1β production is higher in patients with uncontrolled hyperlipidemia, on no or low-dose statin therapy, or with complex plaque imaging features.
-
•
The present study lends support to high-intensity cholesterol lowering and anti-IL-1-directed therapies for patients at high cardiovascular risk.
Summary
CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) confirmed interleukin (IL)–1β as an appealing therapeutic target for human atherosclerosis and related complications. However, there are serious gaps in our understanding of IL-1 production in atherosclerosis. Herein the authors show that complex plaques, or plaques derived from patients with suboptimally controlled hyperlipidemia, or on no or low-intensity statin therapy, demonstrated higher recruitable IL-1β production. Generation of mature IL-1β was matched by IL-1α release, and both were attenuated by inhibition of NLR family pyrin domain containing 3 or caspase. These findings support the inflammasome as the main pathway for IL-1α/β generation in atherosclerosis and high-intensity lipid-lowering therapies as primary and additional anti-IL-1-directed therapies as secondary interventions in high-risk patients.
The innate immune cytokine interleukin (IL)–1β plays key roles in an extended spectrum of inflammatory conditions, including atherosclerosis and its complications. IL-1β blockade in patients with atherosclerosis reduces the burden of inflammation and recurrence of cardiovascular events, establishing an important role for IL-1 signaling in the pathogenesis of atherosclerosis (1).
Generation of mature IL-1β is a dynamic process controlled by inflammasome activation. In the context of atherosclerosis, cholesterol crystals and oxidized low-density lipoprotein (LDL) have the properties of danger signals that can activate the nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing protein (NLRP) 3 inflammasome in macrophages 2, 3, 4, leading to caspase-1-mediated IL-1β maturation and secretion. Alternatively, IL-1β generation can be induced in macrophages by lipopolysaccharide (LPS) via activation of caspase-4 5, 6. In a prior, primarily messenger ribonucleic acid (mRNA) profiling–based study, our group demonstrated the expression of the NLRP3 inflammasome and IL-1β in atherosclerotic plaques and provided initial evidence that IL-1β can be released in response to cholesterol crystals (5). Despite these advances, important gaps remain in our understanding of the generation of IL-1β and the regulation of inflammasome function in human arthrosclerosis.
Herein, using a plaque explant culture system that retains much of the cellular connections and functions in a near-native microenvironment and extending to a larger cohort of patients at 2 different study sites in Europe and the United States, we investigated the biology of inflammasome-mediated IL-1 signaling in human atherosclerotic plaques, including the connection with clinical parameters such as hyperlipidemia and clinical plaque imaging parameters that allow for a correlation with plaque complexity. The present study shows that progression of atherosclerosis is linked to up-regulation of lesional inflammasome activity and NLRP3-mediated generation of IL-1β and IL-1α. These findings provide novel insights into the pathogenesis of atherosclerosis and the therapeutic merit of high-intensity lipid lowering and anti-IL-1 therapy.
Methods
Gene expression analysis
Microarray-based unbiased gene expression analysis was performed on total ribonucleic acid obtained from 127 carotid arterial atherosclerotic plaques and from 10 macroscopically nonatherosclerotic biopsies of the iliac artery and the aorta in the BiKE (Biobank of Karolinska Carotid Endarterectomies) as described previously 7, 8, 9. The full data of the transcriptome analysis are available in Gene Expression Omnibus (accession number GSE21545). All biopsies were collected with written consent from all participants according to the Declaration of Helsinki and with the approval of the Ethical Committee of Northern Stockholm.
Ex vivo culture of atheroma-derived tissue and cells
Ex vivo atheroma cultures were set up as previously described (10). In brief, atheromatous plaques were cut into small pieces (about 1.0 mm3), and calcified tissue was separated out by centrifugation. The decalcified tissue was washed with cold phosphate-buffered saline (PBS) prior to transfer to culture. In some experiments, an enzymatic digestion procedure with collagenase type I (400 U/ml), elastase type III (4 U/ml), deoxyribonuclease (300 U/ml), trypsin inhibitor (1 mg/ml), and polymyxin B (2.5 μg/ml) in 10% fetal calf serum Roswell Park Memorial Institute medium was performed for cell isolation. Atheromatous tissue or isolated cells were distributed on 48-well plates with about 0.1 g tissue/well or 5,000 cells/well. After 2 h incubation in Roswell Park Memorial Institute medium with 10% fetal calf serum, plaque tissues or cells were treated for 24 h with 100 ng/ml LPS (serotype O111:B4, Enzo Life Sciences, Farmingdale, New York), 30 min with 5 mmol/L adenosine 5′-triphosphate disodium salt hydrate (ATP, Sigma-Aldrich, St. Louis, Missouri), or 4 h with 10 μmol/l Salmonella typhimurium (InvivoGen, San Diego, California) or 10 μg/ml poly(deoxyadenylic-deoxythymidylic acid) (LyoVec, InvivoGen). In some experiments, MCC950 (PZ0280, Sigma-Aldrich) or the caspase inhibitor Z-Val-Ala-Asp fluoromethyl ketone (ALX-260-154-R100, Enzo Life Sciences) was applied at indicated concentrations 2 h prior to adding LPS or ATP. Thereafter, supernatants and tissues and cells were snap-frozen and kept at −80°C.
Functional atherosclerotic plaque studies
Functional characterization of IL-1 production (and inflammasome activity) in atherosclerosis was conducted on: 1) 17 plaques obtained from patients who underwent carotid or femoral endarterectomy at the Karolinska University Hospital; and 2) 24 carotid artery plaques collected at the time of carotid endarterectomy as part of an ongoing repository at the Mayo Clinic Rochester, approved by the Mayo Foundation Institutional Review Board. Written informed consent was obtained from all participants. As previously described 11, 12, the decision to undertake surgical intervention followed clinical guidelines and included symptomatic and asymptomatic plaques. Demographic data, detailed clinical history, and imaging data were obtained for each patient by chart review with attention to cerebral ischemic events, cardiovascular risk factors, and medications. As part of the clinical evaluation and surgical planning, all patients at the Mayo Clinic underwent carotid artery imaging. The type of study (i.e., carotid ultrasound with Doppler, carotid computed tomographic angiography, or magnetic resonance angiography) was at the discretion of the providers and was conducted per routine clinical practice standards. The studies were read by board-certified radiologists, documented as clinical radiology reports, and reviewed for the diagnosis of specific plaque features such as ulceration, calcification, and hemorrhage.
Enzyme-linked immunosorbent assay
IL-1β and IL-1α in supernatant were measured using a standard commercial (DY200 for IL-1α and DY201 for IL-1β, R&D Systems, Minneapolis, Minnesota) and a high-sensitivity (HSLB00D for IL-1β, R&D Systems) enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions.
Lesion composition analysis, immunohistochemistry and immunofluorescent staining
Twenty-two carotid plaques were randomly selected from the BiKE study for lesion composition analysis. Macrophages and NLRP3 inflammasome were identified in consecutive sections by immunohistochemistry using primary antibodies to NLRP3 (AG-20B-0014, 1:200, AdipoGen Life Sciences, San Diego, California) and CD68 (HPA048982, Atlas Antibodies, Bromma, Sweden). The presence of iron was determined using Perls’ Prussian blue stain as a sign of hemorrhage. Picrosirius red staining was used for the detection of collagen fibers and fibrous cap thickness. Toluidine blue staining and alizarin red staining were used to evaluate necrosis and calcium deposition, respectively. Quantification of staining was documented as threshold area divided by lesion area using QWin Standard Y 2.8 (Leica, Wetzlar, Germany) computerized analysis. The investigated endarterectomy samples were ranked according to the results. Vulnerability index was defined by the ranks of CD68 and Perls’ Prussian blue staining divided by the ranks of fibrous cap thickness.
Additionally, for immunofluorescent staining, sections of human carotid plaque and internal mammary artery were deparaffinized and rehydrated. Antigen retrieval was performed using Diva Decloaker (DV2004 MX, Biocare Medical, Pacheco, California) at 60°C overnight. The slides were then washed with PBS and blocked in avidin/biotin blocking kit (SP-2001, Vector Laboratories, Burlingame, California) for 30 min and then 5% normal horse serum in PBS with Tween 20 for 30 min. They were stained subsequently with primary antibodies diluted in 5% normal horse serum in PBS with Tween 20 and incubated at 4°C overnight, followed by incubation with a fluorochrome-conjugated secondary antibody (DI 2594, DI 1594, DI 2488, and DI 1488, 1:300, Vector Laboratories) in 5% normal horse serum in PBS with Tween 20 for 1 h. The following primary antibodies were used: NLRP3 (AG-20B-0014, 1:200), apoptosis-associated speck-like protein containing a CARD (ASC) (AG-25B-0006, 1:150, AdipoGen Life Sciences), CD68 (HPA048982, 1:400) and CD68 (NCL-L-CD68, 1:50, Leica Biosystems, Wetzlar, Germany). Immunofluorescent staining was analyzed using a laser scanning confocal microscope (TCP II, Leica). High-resolution images were taken under 40×/1.25 NA and 63×/1.4 NA oil-immersion objective lenses.
Western blot analysis
Plaque-derived cell lysates and supernatants were used for Western blot analysis. The protein samples (200 μg/well from cell lysates and 15 μl from supernatants) were resolved on 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membrane using a wet-transfer system. Membranes were blocked in 10% wt/vol dried milk in TBST (50 mmol/l Tris/HCl [pH 7.6], 150 mmol/l NaCl, and 0.1% vol/vol Tween 20) for 1 h at room temperature. Membranes were incubated with primary antibodies diluted in 5% vol/vol bovine serum albumin in TBST at 4°C overnight and then with the appropriate horseradish peroxidase–conjugated secondary antibody diluted in 5% wt/vol dried milk in TBST for 1 h. Membranes were developed using ECL (Bio-Rad, Hercules, California) and stripped using ReBlot kit (Millipore, Billerica, Massachusetts) before being reprobed. Primary antibodies used were IL-β (#2021, 1:1,000, Cell Signaling Technology, Danvers, Massachusetts) and GAPDH (ab181602, 1:20,000, Abcam, Cambridge, United Kingdom).
Statistical analysis
Data are presented as mean ± SEM unless mentioned otherwise. Differences in mRNA abundances or IL-1 concentrations between groups were tested using the Mann-Whitney U test, Wilcoxon matched-pairs test, or Welch’s t test, with p values as reported. Pearson correlation was applied for statistical analysis of the relationships between plaque IL-1 activity and clinical parameters. Associations between NLRP3 staining, plaque composition, and vulnerability index were assessed using Spearman’s rank correlation coefficient test. A p value <0.05 was considered to indicate statistical significance.
Results
Symptomatic atherosclerotic plaques are associated with up-regulation of selective inflammasome pathways
Analysis of the expression of inflammasome-related genes in the BiKE registry revealed significant changes in the inflammasome profile associated with atherosclerosis development, characterized by marked increases in canonical inflammasome (NLRP1-3[13], NLRP8-9, NLRP11-12, nucleotide-binding oligomerization domain, leucine-rich repeat and CARD domain-containing protein [NLRC3-5], nucleotide-binding oligomerization domain, leucine-rich repeat and BIR domain–containing protein, Pyrin, and absent in melanoma 2) and noncanonical inflammasome components (caspase-4, caspase-5) compared with microscopically nonatherosclerotic iliac arterial specimens (Figure 1A). Moreover, although most inflammasome components were similar between symptomatic plaques and asymptomatic plaques, NLRP6, NLRP12, NLRC4, NLRP3, and caspase-4 transcripts were distinctively enriched in symptomatic plaques (Figure 1B). These data highlight a correlation between the expression of multiple inflammasome elements, atherosclerosis, and plaque vulnerability.
Figure 1.
Transcriptional Profiling of Inflammasome-Interleukin Pathways in Human Atherosclerotic Plaques
(A) Heat map representation of differentially expressed genes in inflammsome-interleukin-1 pathways in atherosclerotic plaques (atheroma, n = 125) obtained from patients undergoing carotid endarterectomy and in nondiseased arteries (control, n = 10) from organ donors. (B) Heat map representation of the top 5 differentially expressed inflammasome genes in atherosclerotic plaques from patients without clinical symptoms (asymptomatic, n = 40) and patients with clinical symptoms (symptomatic, n = 85). Gene expression was determined by ribonucleic acid microarray analysis. P values are based on Mann-Whitney U test. The scale bar shows color-coded differential expression, with red indicating higher levels of expression and blue indicating lower levels of expression. AIM2 = absent in melanoma 2; ASC = apoptosis-associated speck-like protein containing a CARD; CASP = caspase; IL = interleukin; NAIP = nucleotide-binding oligomerization domain, leucine-rich repeat and BIR domain–containing protein; MEFV = Mediterranean fever; NLRC = nucleotide-binding oligomerization domain, leucine-rich repeat and CARD domain–containing protein; NLRP = nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing protein.
Alternative IL-1 signaling pathways in atherosclerosis
Given its significance for the generation of IL-1 in atherosclerosis (13), we further investigated the expression and activity of the NLRP3 inflammasome in human atherosclerosis. NLRP3 protein was detected ubiquitously in all advanced atherosclerotic lesions, primarily in lesional macrophages (Figure 2A) though rarely seen in microscopically nonatherosclerotic arteries (Figure 2B). This supports the notion that NLRP3 is negatively regulated by post-transcriptional modifications in healthy tissues at steady states (14). Besides NLRP3, ASC, the inflammasome adaptor protein, was also highly expressed in lesional macrophages (Figure 2C). However, the aggregate of NLRP3-ASC, indicative of the formation of activated NLRP3 inflammasomes, was rarely observed in atherosclerotic lesions (Figure 2C).
Figure 2.
Alternative Inflammasome Pathways in Atherosclerotic Lesions
(A,B) Confocal microscopy of carotid plaque sections (left) stained for nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing protein (NLRP) 3 and CD68-positive macrophages (left) or internal mammary artery sections (right) stained for NLRP3 and SMC (SMA). Images are representative of carotid atherosclerotic plaques and internal mammary artery specimens. (C) Confocal microscopy of apoptosis-associated speck-like protein containing a CARD (ASC), NLRP3, and macrophages dual staining in adjacent sections of atherosclerotic plaque as shown in B. (D to F) Interleukin (IL)-1β production by atherosclerotic plaque samples in response to NLRP3 activators adenosine 5′-triphosphate disodium salt hydrate (5 mmol/l), nucleotide-binding oligomerization domain, leucine-rich repeat and CARD domain–containing protein (NLRC) 4 activator Salmonella typhimurium (S. typh) (10 μmol/l), or absent in melanoma 2 activator poly(deoxyadenylic-deoxythymidylic acid) (poly dA dT) (10 μg/ml). The concentration of IL-1β in the supernatant was quantified using enzyme-linked immunosorbent assay (ELISA). Data are shown as mean ± SEM; n = 3 to 7. ns, not significant (Mann-Whitney U test). (G,H) Release of IL-1 cytokines from atherosclerotic plaque samples upon lipopolysaccharide (LPS) challenge (100 ng/ml for 24 h). IL-1α (n = 16) and IL-1β (n = 24) concentrations in the supernatant were measured by ELISA. P values are based on Mann-Whitney U test. Ctrl = control; DAPI = 4′,6-diamidino-2-phenylindole.
On the basis of the gene expression data shown in Figure 1, we postulated that there are likely additional inflammasome pathways with a functional role in atherosclerosis. Assessing IL-1β release from atheromatous tissues in response to the known canonical inflammasome activators, we found that IL-1β generation was increased, albeit not statistically significantly, upon exposure to the NLRP3 inflammasome activator ATP or the NLRC4 activator S. typhimurium (Figures 2D and 2E), but not the absent in melanoma 2 inflammasome activator poly(deoxyadenylic-deoxythymidylic acid) (Figure 2F). Alternatively, a burst in IL-1β production (38.9 ± 14.3 pg/ml) along with an equivalent quantity of IL-1α release (34.6 ± 16.5 pg/ml) was induced in 71% (17 of 24) and 41% (7 of 16) of plaques, respectively, after 24 h stimulation of atheromatous tissue with LPS, a defined noncanonical inflammasome stimulus (Figure 2E). Overall, 6 plaques (25%) exhibited a superior response to LPS, with an average IL-1β yield of ≥28.8 pg/ml, but 7 plaques (29%) produced minor or undetectable IL-1β (<3.9 pg/ml) in response to LPS. These data incline to the view that besides NLRP3, there are possibly additional inflammasome pathways, including noncanonical inflammasome pathways, with potential functional implications in atherosclerosis.
Increased IL-1β production in patients with suboptimally controlled hyperlipidemia
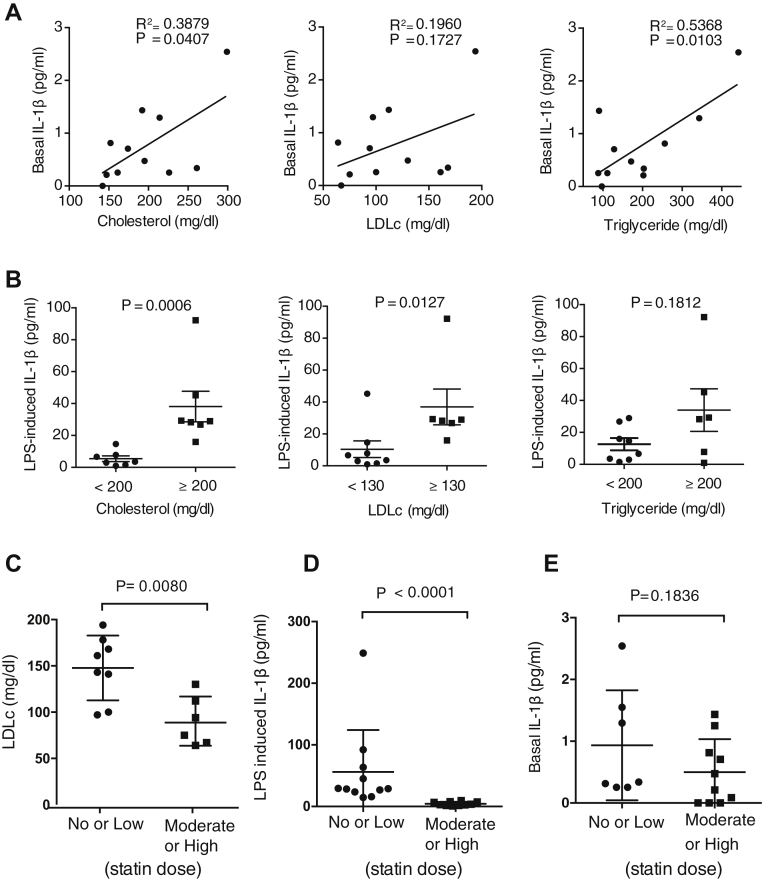
Modulation of immune and inflammation pathways is a central mechanism by which risk factors contribute to atherosclerosis (15). Yet whether and which cardiovascular risk factors affect IL-1 signaling in atherosclerotic plaques are unclear. Herein we analyzed the association between atheromatous tissue IL-1β production and age, sex, body mass index, serum levels of LDL cholesterol and triglycerides, hypertension, diabetes, and time interval from carotid endarterectomy and the latest ischemic cerebrovascular event. This revealed a connection between basal IL-1β production and serum total cholesterol and triglycerides levels (Figure 3A). Notably, the atheromatous tissue from patients with blood LDL cholesterol >130 mg/dl or total cholesterol ≥200 mg/dl yielded a 10-fold higher quantity of IL-1β than plaques from patients with lower blood LDL cholesterol or total cholesterol (Figure 3B).
Figure 3.
Uncontrolled Hypercholesterolemia Is Accompanied by Increased Interleukin-1β Production in Atherosclerotic Plaques
(A) Pearson correlation analysis between unstimulated interleukin (IL)–1β release from atherosclerotic plaque samples and serum levels of total cholesterol, low-density lipoprotein cholesterol (LDLc), and triglycerides (n = 11). (B) Analysis of lipopolysaccharide (LPS)–triggered IL-1β response of atherosclerotic plaque samples in relation to serum levels of total cholesterol, LDLc, and triglycerides. P values are based on Mann-Whitney U test. Data are shown as mean ± SEM; n = 14. (C) Blood LDLc levels in patients with indicated intensity of statin treatment. (D) LPS-induced IL-1β production by ex vivo cultured atheromatous tissue from patients with indicated intensity of statin treatment. (E) Baseline IL-1β production by ex vivo cultured atheromatous tissue from patients with indicated intensity of statin treatment. In C to E, data are shown as mean ± SEM; n = 14 to 24. The p values are based on Mann-Whitney U test.
We furthermore investigated plaque IL-1β generation in relation to blood cholesterol management. Analysis of blood LDL cholesterol levels in 21 patients and their statin therapy history showed that the average level of circulating LDL cholesterol was significantly higher in patients who received no or low-intensity statin therapy compared with patients who received moderate- or high-intensity stain therapy, on the basis of the American College of Cardiology/American Heart Association guideline (Figure 3C). Importantly, LPS-induced IL-1β generation was reduced in atheromatous tissues from patients on moderate- to high-intensity statin therapy (Figure 3D).
Increased IL-1β generation in complex plaques
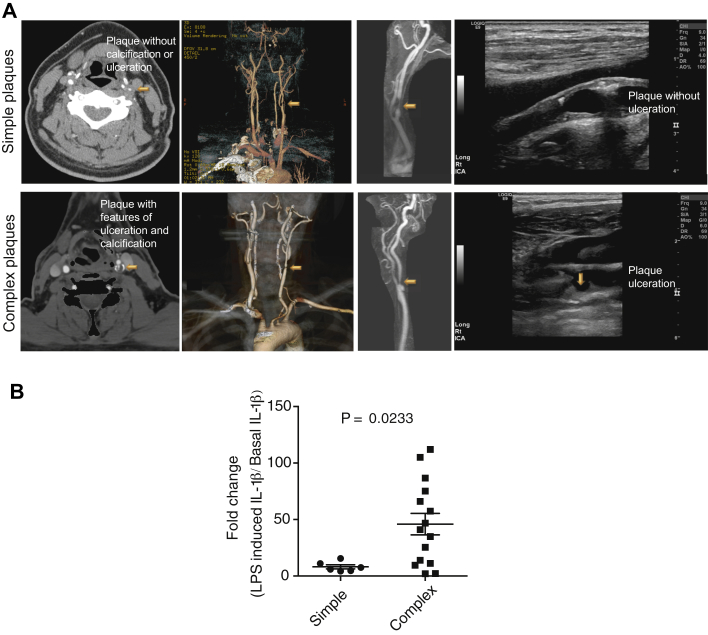
To investigate pathogenic activity of IL-1 in relation to plaque progression, we assessed the difference in IL-1β generation between atherosclerotic plaques with or without imaging signs of calcification, hemorrhage, or ulceration (16). Among the 21 consecutive Mayo Clinic patients who underwent carotid plaque imaging without exclusions by either computed tomography, ultrasound, and/or magnetic resonance imaging, severe calcification, intraplaque hemorrhage, or ulceration, alone or combined, was reported in 15 (72%) cases, herein referred to as complex plaques (Figure 4A). Assessment of ex vivo cultured atheromatous tissues did not reveal statistically significant differences between simple and complex plaques in terms of basal IL-1β generation (data not shown). Nonetheless, the IL-1β yield in response to LPS was increased approximately 45-fold from baseline in tissues from complex plaques, compared with an 8-fold increase in tissues from simple plaques (Figure 4B).
Figure 4.
Enhanced Interleukin-1β Signaling in Complex Atherosclerotic Plaques
(A) Representative computed tomographic angiographic imaging (left), magnetic resonance angiographic imaging (middle), and ultrasound imaging (right) of simple (top) and complex (bottom) atherosclerotic plaques of human internal carotid arteries. (B) Lipopolysaccharide (LPS) (100 ng/ml)–induced interleukin (IL)–1β production relative to basal IL-1β production in atherosclerotic plaque samples isolated from simple (n = 6) and complex (n = 15) carotid atherosclerotic plaques. The concentration of IL-1β in the supernatant was determined using enzyme-linked immunosorbent assay. The p values are based on Wilcoxon matched-paired signed rank test.
Up-regulation of NLRP3 inflammasome expression is a distinctive characteristic of progressive atherosclerosis
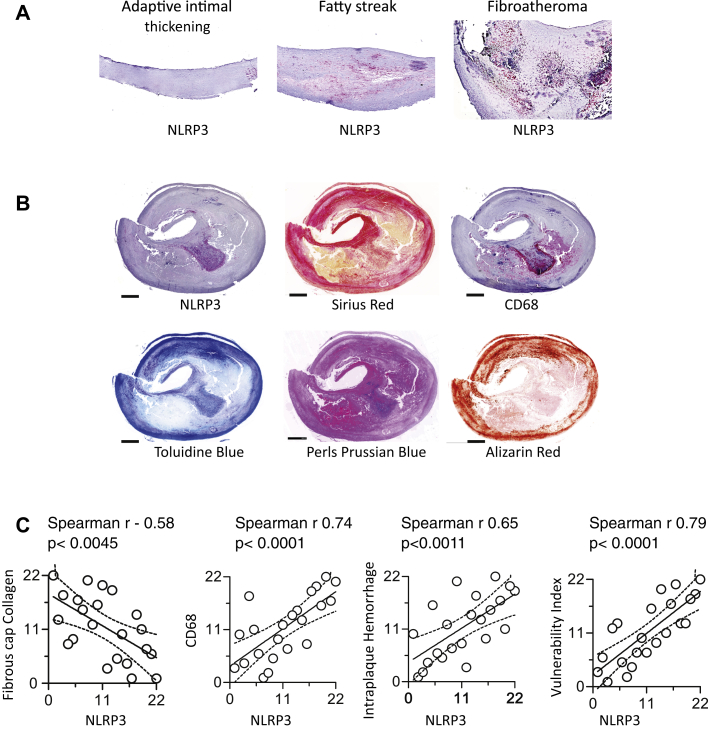
Given the role of NLRP3 observed in experimental atherosclerosis and enhanced IL-1 signaling in complex plaques, we further analyzed the relevance of NLRP3 inflammasome for plaque pathological alterations in humans. NLRP3 expression was noted first in fatty streaks and increased markedly in fibroatheroma (Figure 5A). In a series of 22 carotid plaques collected in the context of the BiKE study, we furthermore noted that NLRP3 inflammasome expression correlated positively with lesion macrophages and intraplaque iron content (a sign of plaque hemorrhage) and inversely with fibrous cap thickness (a sign of plaque stability) (Figures 5B and 5C). Overall, the analyses link the increased NLRP3 expression to pathological features of plaque vulnerability and complexity (Figure 5C).
Figure 5.
NLRP3 Expression Along the Progressive Stages of Atherosclerosis and Complex Plaques
(A) Immunohistochemical characterization of nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing protein (NLRP) 3 expression and its correlation with the progression from adaptive intimal thickening to fatty streak and fibroatheroma in human carotid arteries. (B) Representative images of complex fibroatheromata in human carotid arteries stained for NLRP3 protein (NLRP3), fibrous cap collagen (Sirius red staining), macrophages (CD68), and intraplaque hemorrhage (Perl’s Prussian blue staining). (C) Spearman rank correlation analysis of associations between lesional NLRP3 and fibrous cap collagen, macrophage content, intraplaque hemorrhage, and plaque vulnerability. Data are presented as ranks of corresponding measurement in numeric order (n = 22).
Concomitant release of IL-1α and IL-1β in atherosclerosis
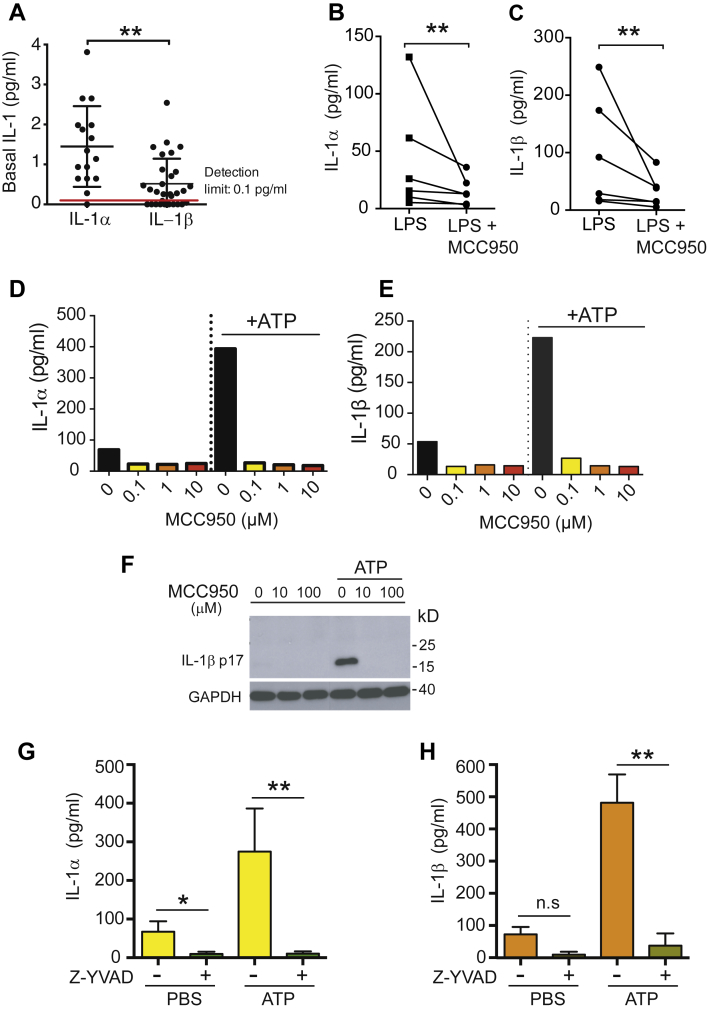
In contrast to IL-1α, the biology of IL-1β signaling in atherosclerosis remains elusive. Previous studies in mouse bone marrow–derived macrophages showed that activation of the inflammasome effector caspase-1 is implicated in the secretion of both IL-1α and IL-1β 17, 18, 19, yet whether a similar mechanism is implicated in IL-1α/β secretion in human atherosclerotic plaques remains understudied. Analyzing IL-1β production in the absence of extra stimuli, we unexpectedly found that atheromatous tissues produce not only IL-1β but also IL-1α constitutively (Figure 6A). Applying MCC950, an NLRP3 inhibitor (20), to atherosclerotic plaque–derived tissues, we corroborated whether the NLRP3 is required for IL-1α and IL-1β secretion in atherosclerotic lesions. We observed (Figures 6B and 6C) that exposure of human atherosclerotic plaque–derived tissue to MCC950 (at 0.1 μmol/l) significantly reduced both IL-1β and IL-1α release triggered by LPS (Figure 6A). Likewise, MCC950 at the same concentration was also capable of preventing basal as well as ATP-induced secretion of IL-1α and IL-1β by plaque-derived cells, composed of 30% to 40% macrophages, 10% to 20% smooth muscle cells, and 5% to 15% T cells according to a previous report (Figures 6B and 6C) (21). Similar to MCC950, IL-1α/β release under steady state conditions or in response to ATP was also impeded by Z-Val-Ala-Asp fluoromethyl ketone, a caspase-1 inhibitor (Figures 6E and 6D). These observations support the view that release of both cytokines, IL-1β and IL-1α, is likely determined by the NLRP3-caspase-1 pathway in atherosclerotic plaques.
Figure 6.
NLRP3 and Caspase-1-Dependent Generation of Interleukin-1α and Interleukin-1β in Atheroma
(A) Release of interleukin (IL)–1α and IL-1β from carotid atherosclerotic plaque samples cultured ex vivo for 20 h in the absence of external inflammasome stimuli. Supernatant concentrations of IL-1α (n = 16) and IL-1β (n = 24) were quantified using high-sensitivity enzyme-linked immunosorbent assay (ELISA). (B,C) ELISA assessment of lipopolysaccharide (LPS)–induced IL-1α and IL-1β release from carotid atherosclerotic plaque samples untreated or pre-treated with MCC950 (100 nmol/l) (n = 6); Wilcoxon matched-pairs test. (D,E) ELISA assessment of basal and adenosine 5′-triphosphate disodium salt hydrate (ATP)–induced IL-1α and IL-1β in the supernatant of ex vivo culture of carotid atherosclerotic cells with or without MCC950. Data are representative of 2 independent experiments. (F) Western blot analysis of basal and ATP-induced IL-1β release from atherosclerotic plaque–derived cells (5,000 cells/well) in the absence or presence of MCC950 (10 μmol/l). Results are representative of 2 independent experiments. (G,H) ELISA assessment of basal and ATP-induced IL-1α and IL-1β release from atherosclerotic plaque–derived cells (5,000 cells/well) in the absence or presence of caspase-1 inhibitor Z-Val-Ala-Asp fluoromethyl ketone (Z-YVAD). Data are shown as mean ± SEM; n = 3 to 10. Welch corrected t test. n.s, not significant; PBS = phosphate-buffered saline.
Discussion
The present study shows that: 1) NLRP3 expression increases with plaque progression and complexity; 2) NLRP3/caspase-1 inhibition blocks IL-1β and IL-1α release from atherosclerotic plaque–derived tissue and cells; 3) human atherosclerotic plaques produce IL-1β and IL-1α constitutively and with a several-fold increase upon stimulation with inflammasome activators; and 4) recruitable IL-1β and IL-1α release is higher in patients with suboptimally controlled hyperlipidemia, on no or low-intensity statin therapy, or with complex plaque imaging features. These findings confirm and extend prior experimental studies and lend translational support for optimal lipid control, high-intensity statin therapy, and anti-IL-1β therapies in patients with ASCVD, especially in those who had clinical events.
In a prior study, increased transcripts of NLRP3, ASC, caspase-1, and IL-1β and IL-18 were found in carotid atherosclerotic plaques (5). Herein we expanded the mRNA profiling studies with the generation of heat maps that more clearly show: 1) the profound differences between nondiseased and atherosclerotic vessels; 2) how many members of the inflammasome family are expressed in atherosclerotic plaques; and 3) how much “hotter” the expression is in symptomatic plaques in particular. Importantly, these genetic data provide a link between multiple inflammasome elements, atherosclerosis, and its activity. They furthermore suggest that apart from NLRP3, there are possibly additional inflammasome pathways relevant to IL-1 signaling in atherosclerosis. This is furthermore underscored by markedly elevated levels of NLRC4 in the plaque of symptomatic patients and plaque IL-1β production in response to external NLRC4 ligands. Additionally, increased expression of caspase-4 in atherosclerotic plaques and generation of IL-1β by plaque-derived tissue upon LPS stimulation point also into the direction of noncanonical inflammasome activation 5, 22, an alternative mechanism that has been demonstrated for IL-1α secretion in murine macrophages in vitro but has not been shown in human atherosclerotic plaques (19).
The presence of both IL-1α and IL-1β was recently observed in human atherosclerotic plaques (23). Taking the advantage of ex vivo culture of atherosclerotic plaque–derived tissue, our study provides further evidence that human atherosclerotic plaques secrete both IL-1 cytokines with similar kinetics under steady state conditions and in response to external stimuli. These independent observations suggest that both IL-1α and IL-1β may participate in atherosclerosis. In line with this notion is the observation that genetic deficiency of IL-1α, even if confined to bone marrow–derived cells, mitigates atherosclerotic burden in a mouse model. This protective effect was even more pronounced when combined with depletion of IL-1β (24). Thus, various lines of research point to the biological importance of both IL-1α and IL-1β for the atherosclerotic disease process. Given that atherosclerotic plaques retain a sufficient capacity of IL-1α production and functional similarities between the 2 cytokines, uncontrolled IL-1α generation can be as important as IL-1β in the pathogenesis of atherosclerosis.
Exploring the mechanisms contributing to IL-1α/β signaling in atherosclerotic lesions, we discovered that treatment of tissues or cells isolated from atheroma with the NLRP3-specific inhibitor MCC950 (20) or the caspase-1 inhibitor Z-Val-Ala-Asp fluoromethyl ketone inhibited the release of both IL-1α and IL-1β under ATP/LPS-stimulated and unstimulated baseline conditions. These findings lead to the hypothesis that NLRP3 inflammasome is a main determinant of IL-1β and IL-1α signaling in atherosclerosis. Mechanistically, ATP or LPS induce the processing of IL-1β and IL-1α by activation of caspase-1 via the NLRP3 inflammasome or noncanonical inflammasome pathways and the release of these ILs through gasdermin-D-formed pores 19, 25, 26. Alternatively, the NLRP3-caspase-1 pathway may have a dual mechanism of action, producing mature IL-1β and facilitating IL-1α/β secretion, suggesting that targeting NLRP3-caspase-1 may yield better outcomes than targeting the 2 IL-1 isoforms in separation. In keeping with an experimental in vivo study, noting a reduction in vascular inflammation and atherosclerotic lesion development by inhibition of the inflammasome with MCC950 (27), targeting the NLRP3 inflammasome may offer a new approach to mitigate IL-1-driven inflammatory responses in atherosclerosis.
Along these lines, the present results support CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) (1), which showed that the IL-1β-neutralizing antibody canakinumab reduced the primary combined endpoint of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death among patients with coronary artery disease and a history of myocardial infarction and elevated C-reactive protein levels despite aggressive secondary prevention measures. Finally, these data shed light on the link between an acutely and subacutely increased risk for acute ischemic events such as myocardial infarction and stroke in the setting of acute infections (28). The endogenous production of LPS under these circumstances may very well stimulate IL-1 production in human atherosclerotic plaques in vivo, with an increase in plaque inflammation and subsequent plaque destabilization. The convergence of these investigations underscores the significance of IL-1β-driven inflammatory responses in atherosclerosis.
An important observation of the present study is that IL-1β release kinetics relate to serum lipid levels. A linear correlation was seen between IL-1β production at baseline and total cholesterol and triglyceride levels. LPS-induced IL-1β release was significantly higher in patients with cholesterol levels >200 mg/dl and LDL levels >130 mg/dl. LDL cholesterol overload, oxidation of LDL, and fatty acids were shown before to induce a continuous state of intracellular stress with up-regulation of IL-1 and NLRP3 inflammasome expression in macrophages 4, 29. However, the current analysis of the BiKE study, a large-scale mRNA transcriptional database of human carotid atherosclerotic plaques, did not substantiate a connection between elevated levels of systemic LDL cholesterol and the local inflammasome and IL-1 transcription in atherosclerotic plaque (data not shown). LDL cholesterol and oxidized LDL are implicated in training monocytes through epigenetic reprogramming to acquire a long-lasting proinflammatory phenotype with enhanced inflammasome activity (30). Alternatively, uncontrolled blood LDL cholesterol may lead to increased cholesterol crystal formation and noncrystalline cholesterol overload in atherosclerotic lesions, thus accounting for an increased risk of inflammasome activation and consequently IL-1 production (5). Such considerations lend support to the benefits of high-intensity lipid-lowering therapy. Indeed, in the present study, we did find that atherosclerotic plaque tissues from patients on no or low-intensity statin therapy mounted a much higher IL-1β production upon stimulation. Although it cannot be excluded that non-lipid-lowering properties contributed to this observation, a correlation between LDL levels and statin therapy intensity was seen. In combination, these studies extend our understanding of the link between hyperlipidemia, inflammasome activity, and inflammation in atherosclerosis and support aggressive lipid-lowering therapy as an important translational aspect.
The present investigation also reveals marked heterogeneity in inflammasome activity among atherosclerotic plaques. Notably, complex lesions with imaging signs of hemorrhage, ulceration, or calcification mounted a 10-fold higher induction of IL-1β release than simple plaques. In keeping with increased expression of inflammasome components in symptomatic plaques, these data associate biologically active plaques with increased inflammasome activity. Indeed, up-regulation of the NLRP3 inflammasome in atherosclerotic plaques may relate to many aspects of disease pathogenesis such as vascular inflammation, intraplaque hemorrhage, plaque compositions, and vulnerability. In agreement with the present results, prior studies noted a correlation between elevated circulating levels of IL-1β, coronary calcium burden, and cardiac death (31).
Conclusions
The present study supports the concept that the NLRP3 inflammasome contributes to plaque IL-1α/β generation and provides biological insights into the clinical merit of anti-IL-1 signaling–directed and high-intensity lipid-lowering therapies in high-risk patients with atherosclerosis.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Hypercholesterolemia is the cardinal risk factor and inflammation the characteristic trait of atherosclerosis directing the clinical course. The 2018 American College of Cardiology/American Heart Association guidelines recommend high-intensity statin therapy and lower cholesterol goals for very high risk patients. The CANTOS trial indicated that IL-1β-directed therapy with a neutralizing antibody reduces recurrent ischemic events and cardiovascular death among patients with coronary artery disease and a history of myocardial infarction and elevated C-reactive protein levels despite aggressive secondary prevention measures. Herein we show that complex carotid artery plaques have a higher recruitable production of IL-1β. Similarly, plaques from patients on no or low-intensity statin therapy or with suboptimally controlled hyperlipidemia (LDL level >130 mg/dl) mount higher IL-1β release upon stimulation. Plaque generation of IL-1β is also paired with release of IL-1α and entails likely both the canonical and noncanonical inflammasome pathway. Overall the present study findings underscore a link between plaque inflammatory biological activity and hyperlipidemia as well as plaque complexity.
TRANSLATIONAL OUTLOOK: The results of the present study are supportive of the CANTOS trial by indicating that an IL-1β-directed approach would be most efficacious in patients with a vulnerable phenotype (complex plaques) and those with suboptimal secondary prevention measures. As indicated by the present findings, control of hypercholesterolemia and moderate- to high-intensity statin therapy translate into lower plaque interleukin-1 production and related inflammatory activity. Lipid-lowering and statin therapy intensification efforts should come first with additional anti-inflammatory therapies, especially those that specifically target the inflammasome-IL-1 axis, in very high risk patients as enrolled in the CANTOS trial. The present study lends support to biological plaque activity–directed treatment efforts and the testing thereof in future clinical trials.
Footnotes
This work was supported by the KI-Mayo collaboration project, the Swedish Research Council, the Swedish Heart-Lung Foundation, European Union Seventh Framework Programme projects AtheroFlux (HEALTH-F2-2013-602222) and VIA (HEALTH-F2-2013- 603131), the Foundation for Strategic Research in Sweden, and the National Institutes of Health (HL116952-04). Dr. Jiang was supported by the Chinese Scholarship Council. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and U.S. Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Joerg Herrmann, Email: herrmann.joerg@mayo.edu.
Zhong-qun Yan, Email: zhong-qun.yan@ki.se.
References
- 1.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 2.Duewell P., Kono H., Rayner K.J. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajamaki K., Lappalainen J., Oorni K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheedy F.J., Grebe A., Rayner K.J. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paramel Varghese G., Folkersen L., Strawbridge R.J. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016;5:e003031. doi: 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaidt M.M., Ebert T.S., Chauhan D. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Folkersen L., van’t Hooft F., Chernogubova E. Association of genetic risk variants with expression of proximal genes identifies novel susceptibility genes for cardiovascular disease. Circ Cardiovasc Genet. 2010;3:365–373. doi: 10.1161/CIRCGENETICS.110.948935. [DOI] [PubMed] [Google Scholar]
- 8.Razuvaev A., Ekstrand J., Folkersen L. Correlations between clinical variables and gene-expression profiles in carotid plaque instability. Eur J Vasc Endovasc Surg. 2011;42:722–730. doi: 10.1016/j.ejvs.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Perisic L., Aldi S., Sun Y. Gene expression signatures, pathways and networks in carotid atherosclerosis. J Intern Med. 2016;279:293–308. doi: 10.1111/joim.12448. [DOI] [PubMed] [Google Scholar]
- 10.Ketelhuth D.F., Rios F.J., Wang Y. Identification of a danger-associated peptide from apolipoprotein B100 (ApoBDS-1) that triggers innate proatherogenic responses. Circulation. 2011;124:2433–2443. doi: 10.1161/CIRCULATIONAHA.111.051599. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann J., Mannheim D., Wohlert C. Expression of lipoprotein-associated phospholipase A(2) in carotid artery plaques predicts long-term cardiac outcome. Eur Heart J. 2009;30:2930–2938. doi: 10.1093/eurheartj/ehp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannheim D., Herrmann J., Versari D. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke. 2008;39:1448–1455. doi: 10.1161/STROKEAHA.107.503193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigano E., Diamond C.E., Spreafico R., Balachander A., Sobota R.M., Mortellaro A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun. 2015;6:8761. doi: 10.1038/ncomms9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afonina I.S., Zhong Z., Karin M., Beyaert R. Limiting inflammation-the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat Immunol. 2017;18:861–869. doi: 10.1038/ni.3772. [DOI] [PubMed] [Google Scholar]
- 15.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 16.Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 17.Kuida K., Lippke J.A., Ku G. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 18.Keller M., Ruegg A., Werner S., Beer H.D. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Gross O., Yazdi A.S., Thomas C.J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Coll R.C., Robertson A.A., Chae J.J. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monaco C., Andreakos E., Kiriakidis S. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayagaki N., Wong M.T., Stowe I.B. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 23.Beltrami-Moreira M., Vromman A., Sukhova G.K., Folco E.J., Libby P. Redundancy of IL-1 isoform signaling and its implications for arterial remodeling. PLoS ONE. 2016;11:e0152474. doi: 10.1371/journal.pone.0152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freigang S., Ampenberger F., Weiss A. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 25.Evavold C.L., Ruan J., Tan Y., Xia S., Wu H., Kagan J.C. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48:35–44.e6. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N., Warming S., Lamkanfi M. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 27.van der Heijden T., Kritikou E., Venema W. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2017;37:1457–1461. doi: 10.1161/ATVBAHA.117.309575. [DOI] [PubMed] [Google Scholar]
- 28.Smeeth L., Thomas S., Hall A., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 29.Dang E.V., McDonald J.G., Russell D.W., Cyster J.G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell. 2017;171:1057–1071.e11. doi: 10.1016/j.cell.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christ A., Gunther P., Lauterbach M.A.R. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018;172:162–175.e14. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceneri N., Zhao L., Young B.D. Rac2 modulates atherosclerotic calcification by regulating macrophage interleukin-1beta production. Arterioscler Thromb Vasc Biol. 2017;37:328–340. doi: 10.1161/ATVBAHA.116.308507. [DOI] [PMC free article] [PubMed] [Google Scholar]