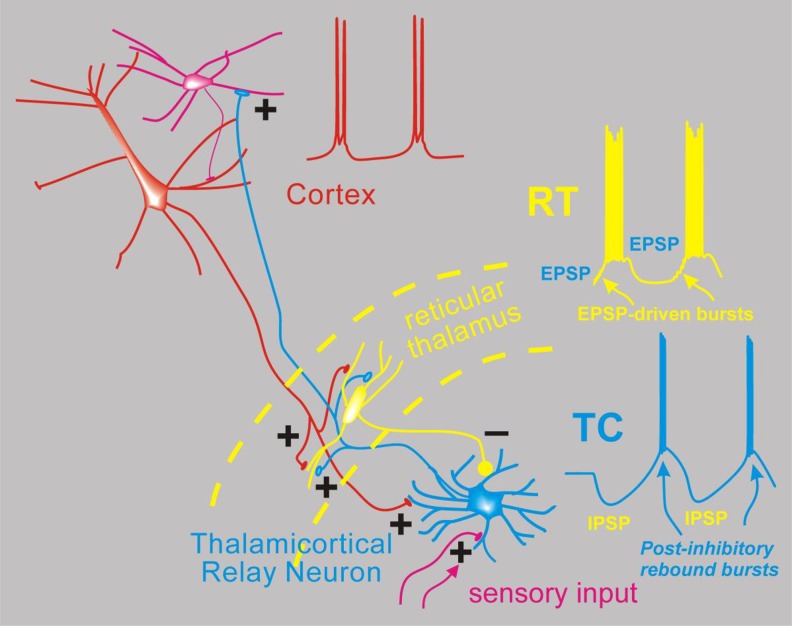
Figure 2.
Simplified network model for absence seizures. Depicted are interconnected neurons of the thalamus and cortex and the spiking activity proposed to occur during spike wave discharge of an absence seizure. From the bottom, thalamocortical (TC) relay neurons (blue) normally receive sensory input in the form of excitatory synapses (+) and transform this into TC cell spikes that propagate the sensory signal to the cortex (red). Thalamocortical neurons emit axon collaterals into the reticular thalamus (RT, yellow), where excitatory synapses activate the resident inhibitory neurons. These then provide feedback inhibition (inhibitory post-synaptic potentials [IPSPs]) to TC cells. During absence seizures, the network presumably becomes synchronized. In this scenario, multiple RT cells fire together producing strong IPSPs onto TC cells, and since TC cells contain high levels of T type calcium channels,8 strong inhibition leads to robust postinhibitory rebound bursts that in turn reactivate both RT and cortex (excitatory blue axonal fibers). Cortical cell firing (red) is also synchronized so that its excitatory output can combine with TC output to strongly activate RT cells, which also contain T-type calcium channels and generate bursts of action potentials via direct synaptic excitation. In this model, cells from all 3 classes (Cortex, TC, RT) actively participate in a near synchronous manner, with the inhibitory output from RT delaying TC cell bursting and thus playing a major role in pacing the epileptic network.