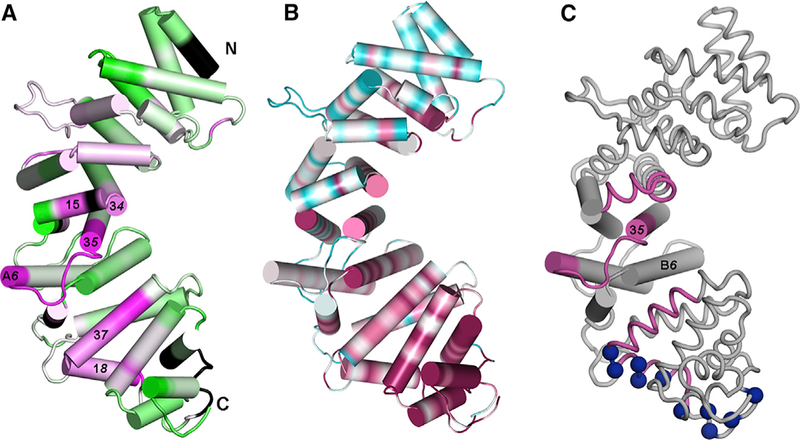
Figure 5. Changes in Hydrogen-Deuterium Exchange at R452 and Amino Acid Sequence Conservation Suggest Sites for Gαi1 Binding.
(A) Segment colored magenta are protected by Gαi1 binding; de-protected segments are colored green. Extent of protection/de-protection indicated by color intensity, to maximum/minimum values of ±16% in change in HDX protection on Gαi1 binding.
(B) Amino acid sequence conservation, computed using CONSURF (Ashkenazy et al., 2010) for 150 Ric-8 homologs, mapped on the surface of pR452. Conservation is depicted over a color range from burgundy (most conserved) to cyan (most variable). Black denotes absence of HDX information.
(C) Spatial relationship between central subdomain, with helices rendered as cylinders and flanking subdomains rendered as coils. Segments that exhibit high HDX protection are colored burgundy and positively charged residues in conserved basic surface are shown as blue spheres at Cα positions. See also Figure S3.