Abstract
Background
Whether continuous positive airway pressure (CPAP) treatment can improve depression or anxiety symptoms in obstructive sleep apnoea (OSA) patients remains uncertain.
Methods
Secondary analysis of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial, combined with a systematic review of randomised evidence. The SAVE secondary analyses involved 2410 patients with co-existing moderate–severe OSA and established cardiovascular disease randomly allocated to CPAP treatment plus usual care or usual care alone and followed up for 3·7 (SD 1·6) years. We evaluated the effect of CPAP treatment on depression and anxiety caseness (scores ≥ 8 on the Hospital Anxiety and Depression Scale depression and anxiety subscales [HADS-D and HADS-A]) for OSA patients.
Findings
CPAP treatment was associated with reduced odds of depression caseness (adjusted odds ratio [OR] 0·80, 95% confidence interval [CI] 0·65–0·98, P = 0·031) compared to usual care in the SAVE trial and the treatment effect was greater in those with pre-existing depression symptoms. A systematic review of 20 randomised trials including 4255 participants confirmed a benefit of CPAP in reducing depression symptoms in OSA patients: the overall effect (standardised mean difference) was − 0·18 (95% CI − 0·24 to − 0·12). No effect of CPAP treatment on anxiety caseness was found both in patients of the SAVE study (adjusted OR 0·98, 95% CI 0·78–1·24, P = 0·89) and the systematic review.
Interpretation
CPAP reduces depression symptoms in patients with co-existing OSA and CVD independently of improvements in sleepiness.
Keywords: Continuous positive airway pressure, Depression, Anxiety, Mood, Obstructive sleep apnoea, OSA, Cardiovascular diseases
Highlights
-
•
Depression and anxiety are prevalent in patients with chronic conditions, such as obstructive sleep apnoea (OSA) and cardiovascular disease, and they adversely impact on prognosis and quality of life. Whether continuous positive airway pressure (CPAP) treatment can improve depression or anxiety symptoms in OSA patients still remains uncertain. We searched PubMed for studies in English language published before 1 Jan 2019 reporting of CPAP effect on depression and anxiety symptoms using the terms “continuous positive airway pressure”, “obstructive sleep apnoea”, “depression” and “anxiety”. A recent systematic review of randomised trials on the effect of CPAP on depression symptoms had found modest benefit of CPAP treatment for improving depression symptoms, however there was high heterogeneity. Existing findings are variable across studies due, in part, to different study designs, small patient numbers, short periods of follow-up, and from an emphasis on depression over anxiety patterns of abnormal mood.
-
•
Added value of this study: Analysis of the Sleep Apnea Cardiovascular Endpoints (SAVE) cohort of over 2400 patients with moderate to severe OSA and concomitant cardiovascular diseases who were followed up for an average of 3.7 years showed that the CPAP reduced depression symptoms within few months of treatment, independently of improvements in daytime sleepiness. The NNT (i.e. 15) for CPAP to prevent depression caseness (as defined by Hospital Anxiety and Depression Scale – Depression subscale score ≥ 8) is comparable to remission rates achieved for some established antidepressant drugs. However, analyses of the SAVE study showed no clear clinical benefit for anxiety symptoms. Systematic review of 20 included randomised controlled studies showed similar results as our SAVE secondary analyses.
-
•
Implications of all the available evidence: Our findings provide further support for the broader beneficial effects of CPAP in those with OSA, and especially those at high cardiovascular risk, where there is the potential for enhanced mood to improve long-term cardiovascular outcomes.
1. Introduction
Depression and anxiety are public health challenges worldwide, contributing an enormous amount of human misery and lost health. Depression and anxiety commonly affect patients with chronic conditions, such as obstructive sleep apnoea (OSA) and cardiovascular (CV) disease, and they adversely impact on prognosis, quality of life and treatment adherence [1], [2], [3], [4], [5]. The relationship between abnormal mood and OSA is complex and bidirectional, with various potential pathophysiological mechanisms including sleep fragmentation, hypoxia, oxidative stress, inflammation and neurotransmitter imbalance [6], [7]. While continuous positive airway pressure (CPAP), the most widely used treatment for OSA, improves subjective symptoms such as daytime sleepiness, the effects specifically on depression and anxiety are uncertain. Two recent systematic reviews of randomised trials on the effect of CPAP on depression and anxiety reported conflicting conclusions [8], [9]. Existing findings are variable across studies due, in part, to different study designs, small patient numbers, short periods of follow-up, and from an emphasis on depression over anxiety patterns of abnormal mood [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24].
The international multicenter Sleep Apnea Cardiovascular Endpoints (SAVE) study assessed the effectiveness of CPAP treatment for the prevention of serious CV events in 2717 patients with co-existing OSA and coronary and/or cerebrovascular CV disease. A secondary aim was to determine whether CPAP reduced depression and anxiety symptoms, and statistically significant reductions were apparent at the end-of-study visit after several years of treatment in addition to usual care, compared to usual care alone [25]. Herein, we provide in-depth analyses of the temporal effects of CPAP on depression and anxiety among SAVE participants, with the aim of defining subgroups of patients who may derive the greatest benefit from treatment and the interaction of CPAP treatment related effects on mood with improved sleepiness. We also sought to quantify the relevance of these findings in the context of a systematic review of the randomised evidence to provide a robust estimate of the effects of CPAP on depression and anxiety symptoms in patients with OSA. We hypothesised that CPAP treatment would lead to reductions in both depression and anxiety symptoms overtime in OSA patients independent of improvements in daytime sleepiness.
2. Methods
2.1. Clinical trial design and participants
We conducted a secondary analysis of the SAVE study which was an international, multi-center, randomised, open, blinded-outcome assessed, controlled trial of the effectiveness of CPAP for OSA for secondary prevention of CV disease, the details of which are outlined elsewhere [25]. In brief, patients with a history of CV disease and moderate to severe OSA (≥ 4% oxygen desaturation index, ≥ 12 events/h [ApneaLink, ResMed]) during an overnight recording were recruited from 89 clinical centres in seven countries between December 2008 and November 2013. Patients were excluded if they had severe oxygen desaturation (< 80% SpO2 for > 10% of the monitoring time), severe daytime sleepiness (Epworth Sleepiness Scale [ESS] score > 15), or predominant central apnoea. Eligible participants were also required to have used an average of at least 3 h per night of sham-CPAP during a one week run-in period. The study was approved by all relevant ethics committees, and written informed consent was obtained from each participant or their appropriate surrogate. The study is registered at ClinicalTrials.gov (NCT00738179).
2.2. Randomisation and interventions
Eligible patients were randomised centrally to receive either CPAP therapy plus usual care (CPAP group) or usual care alone (usual-care group). Randomisation was stratified by site, type of CV disease (cardiac, cerebrovascular, or both), and severity of daytime sleepiness (ESS score < 11 vs. ≥ 11). CPAP group patients were provided with an automated CPAP machine (REMstar Auto, M or PR series, Philips Respironics) that was initially set in automatic mode for one week and thereafter fixed to the 90th percentile of pressure that was calculated by the automated positive airway pressure device from the recorded data. The core sleep laboratory monitored CPAP adherence and all patients had guideline management of CV risk factors and were provided advice on beneficial sleep habits and lifestyle changes to minimise OSA symptoms.
2.3. Procedures and outcomes
Information on demographic and clinical characteristics, medical history, medications, and health behaviours was recorded at the time of enrolment. Anxiety and depression symptoms were assessed at baseline, and at 6-, 24-, 48-, 72-, and 84-month visits (or end of study, whichever came first), using the Hospital Anxiety and Depression Scale (HADS). The HADS is a widely used screening tool for abnormal mood, with subscales HADS-D and HADS-A each containing 7 items relevant to depression and anxiety symptoms, respectively. HADS subscale scores range from 0 to 21 and can be used to categorise mood as: 0–7 ‘non-case’; 8–10 ‘possible case’; and 11–21 for ‘definite case’ of depression or anxiety [26], [27]. For the main analyses, we defined depression and anxiety ‘non-caseness’ as having a HADS score of 0–7; and ‘caseness’ as having a HADS score of 8–21, as a cut-off score of 8 was found to be optimal for best sensitivity and specificity [26].
2.4. Statistical analysis
Baseline patient characteristics were summarised as mean (SD) for continuous variables, and as number (%) for categorical variables, with comparisons between groups made using Wilcoxon or chi-squared tests. Unadjusted logistic regression was conducted to evaluate the relationship between CPAP and depression and anxiety caseness at the end of follow-up, as defined by scores ≥ 8 on each of the HADS-D and HADS-A subscales. Sensitivity analyses were performed with the higher cut-point of ≥ 11 on each of the HADS-D and HADS-A [26]. We also adjusted for the change from baseline to the end of study visit in the ESS score to evaluate whether any CPAP treatment effects on depression and anxiety were independent of changes in sleepiness. Based on the changes in absolute risk of depression and anxiety (HADS-D and HADS-A scores 8–21), we calculated the number needed to treat (NNT) at the end of follow-up [28]. The impact of CPAP adherence on depression and anxiety caseness was evaluated in patients randomised to CPAP treatment using logistic regression adjusted for other confounders. CPAP adherent was defined as having used ≥ 4 h (on average per night over a 48 months period), which is a widely used cut-off in prior studies [8], [29]. Heterogeneity in the CPAP treatment effect on HADS-D and HADS-A was assessed across subgroups defined by age (≤ 65 vs > 65 years) sex, ethnicity (Asian vs non-Asian), body mass index (BMI, < 30 vs ≥ 30 kg/m2), baseline depression and anxiety symptoms (HADS-D and HADS-A scores < 8 vs ≥ 8, respectively), OSA severity (Apnoea Hypopnea Index [AHI] < 30 vs ≥ 30 events/h), daytime sleepiness (ESS scores ≤ 10 vs > 10), history of coronary artery disease and cerebrovascular disease, through the addition of interaction terms to the logistic regression models. Longitudinal changes in mean HADS-D and HADS-A scores by treatment group were visually examined with trajectory plots, and the between-group differences assessed using a linear mixed effects model with group, time, and a group*time interaction as fixed effects; the overall difference between groups in trajectories were assessed using the p-value for the group*time interaction term. We also assessed temporal changes in the individual components of the HADS using similar linear mixed effects models. All tests were two-sided and statistical significance set at P < 0·05. Results are presented with 95% confidence intervals (CI). Analyses were undertaken using SAS 9·0 (SAS Institute, Cary, NC, USA) and STATA 14·2 (StataCorp, College Station, TX, USA).
2.5. Systematic review
The systematic review protocol was registered in PROSPERO (CRD42017078483) in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [30]. Inclusion criteria were all randomised controlled trials with consecutive patient recruitment, including parallel and crossover designs, comparing CPAP to non-active controls (oral placebo, standard care, or sham-CPAP) for mood improvement in adults (≥ 18 years and CPAP-naïve) with clinically diagnosed OSA. The included trials required depressive and/or anxiety symptoms measurement using validated questionnaires/clinical interviews that included the Beck's Depression Inventory (BDI) [31], HADS [27], Montgomery-Åsberg Depression rating scale (MADRS) [32], Profile of Mood States depression subscale [33], or the Geriatric Depression Scale (GDS) [34]. Trials of patients with co-morbid bipolar disorder, schizophrenia, or another psychiatric diagnosis, were excluded because of the potential to compromise CPAP adherence, follow-up, and outcome assessments. Five databases were searched: MEDLINE, EMBASE, PsychINFO, CINAHL and CENTRAL (from inception to August 2018), using adapted versions of the outlined Medline search strategy without language restrictions (Table 1, appendix). Further literature was reviewed from reference lists of key clinical trials and previous systematic reviews [6], [8], [9].
Table 1.
Hospital Anxiety and Depression Scale (HADS) scores among patients with obstructive sleep apnoea and cardiovascular disease at the end of follow-up in the SAVE trial (n = 2410).
| CPAP (Total n = 1220) n (%) |
Usual care (Total n = 1190) n (%) |
Crude OR (95%CI) | P-value | Adjusted OR⁎ (95% CI) |
P-value | |
|---|---|---|---|---|---|---|
| HADS-D† | ||||||
| Any depressive symptoms (score 8–21)‡ | 221 (18·1) | 295 (24·8) | 0·67 (0·55–0·82) | < 0·0001 | 0·80 (0·65–0·98) | 0·031 |
| Depression (score 11–21) | 92 (7·5) | 115 (9·7) | 0·76 (0·57–1·02) | 0·064 | 0·96 (0·71–1·30) | 0·80 |
| HADS-A† | ||||||
| Any anxiety symptoms (score 8–21)§ | 176 (14·4) | 202 (17·0) | 0·83 (0·66–1·03) | 0·086 | 0·98 (0·78–1·24) | 0·89 |
| Anxiety (score 11–21) | 62 (5·1) | 61 (5·1) | 0·99 (0·69–1·42) | 0·96 | 1·24 (0·84–1·81) | 0·28 |
CI denotes confidence interval; CPAP, continuous positive airways pressure; HADS-D Hospital Anxiety and Depression Scale - Depression subscale score; HADS-A Hospital Anxiety and Depression Scale – Anxiety subscale score; SD, standard deviation.
Logistic regression adjusted for change in Epworth Sleepiness Scale score from baseline to end of study.
HADS-A and HADS-D scores range from 0 to 21, with higher scores indicating more severe symptoms.
Number needed to treat to prevent depression caseness (HADS-D score 8–21) for 3·7 years is 15 (95% confidence interval 10 to 31).
Number needed to treat to prevent anxiety caseness (HADS-A score 8–21) for 3·7 years is 39 (95% confidence interval 18 to − 281).
Article screening and data extraction were conducted independently by three authors (DZ, YX, SY) and discrepancies resolved in discussion. Trials were included in the meta-analysis if they reported mean and SD in depression and/or anxiety scores post-intervention in treatment and control groups. We pooled standardised mean differences (and 95% CI) using random effects model of Dersimonian and Laird. Study weights were generated using the inverse of the variance. Between-study heterogeneity was assessed using the I2 statistic in combination together with visual inspection of the forest plots. Subgroup analyses by type of comparator (standard care, oral placebo, and sham-CPAP) also were conducted. The risk of bias assessment was performed by two independent reviewers (YX and XW) using the Cochrane risk of bias assessment tool [35]. Funnel plots and Egger's test were used to assess for publication bias. As the SAVE trial had the largest sample size compared to all other studies, we performed meta-analyses excluding the SAVE study as sensitivity analyses. Meta-analyses and publication bias assessment were conducted using STATA 14·2 (StataCorp, College Station, TX, USA) and risk of bias tables were produced using Revman 5·3 (Revman, The Cochrane Collaboration Oxford, UK).
3. Results
3.1. SAVE study
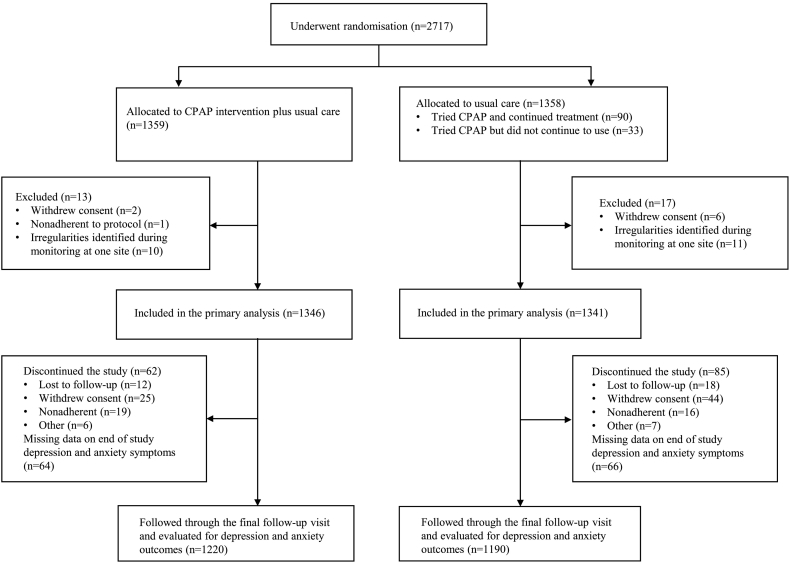
There were 2687 patients of the SAVE study who underwent randomisation (1346 to CPAP intervention and 1341 to usual care alone) and were followed for major CV outcomes for a mean of 3·7 (SD 1.6) years (Fig. 1). For the present analyses, we included 2410 participants who had end of study mood assessment data. Patients included in this study tended to be younger, with higher use of antihypertensive and antithrombotic medication at baseline, and had greater adherence to sham-CPAP during the screening phase of the trial, compared to the 277 patients without end of study mood data who were excluded from these analyses (Table 2, Appendix). However, the included patients were well balanced for characteristics between CPAP treatment and usual care groups (Table 3, Appendix). At the end of study follow-up, 221/1220 (18·1%) individuals in the CPAP group and 295/1190 (24·8%) in the standard care group had ‘depression caseness’ (HADS-D score ≥ 8) (Table 1). The unadjusted OR was 0·67 (95% CI 0·55–0·82; P < 0·0001), and the effect remained significant after adjustment for the change in ESS scores during follow-up (adjusted OR 0·80, 95% CI 0·65–0·98; P = 0·031). In longitudinal analysis of the HADS-D, there was an overall reduction in depressive symptoms (p < 0·0001 for group*time interaction) and significantly fewer subjects with ‘depression caseness’ at most follow-up visits in the CPAP group; the treatment effect being apparent at 6 months post-randomisation (Fig. 1, Table 4, Appendix). Depression caseness with a higher cut-point score ≥ 11 on the HADS-D was also lower in the CPAP compared to usual care group, but the effect was not significant due to small patient numbers (Table 1). The number needed to treat (NNT) in order to prevent one case of depression was 15 (95% CI 10–31). There appeared to be no significant effect of CPAP adherence on depression and anxiety (Table 5, appendix). Subgroup analyses showed that patients with pre-existing depression symptoms had significantly reduced depression symptoms with CPAP treatment (Table 6, appendix). Analyses of each of the questions on the HADS-D showed a consistent effect of CPAP treatment across individual symptom components (Fig. 2, appendix). Anxiety caseness (defined by HADS-A scores ≥ 8) was non-significantly lower in the CPAP group (176/1220, 14·4%) compared to usual care group (202/1190, 17·0%) (Table 1). A trajectory plot of anxiety symptoms (HADS-A score) showed small reductions in anxiety in both usual care and CPAP groups over time, being slightly greater in those assigned to CPAP (Fig. 3, Table 4, appendix). Analyses of the individual questions on the HADS showed that CPAP treatment reduced four individual components of the HADS-A (Fig. 4, appendix).
Fig. 1.
Consort flow diagram
CPAP denotes continuous positive airways pressure; OSA, obstructive sleep apnoea.
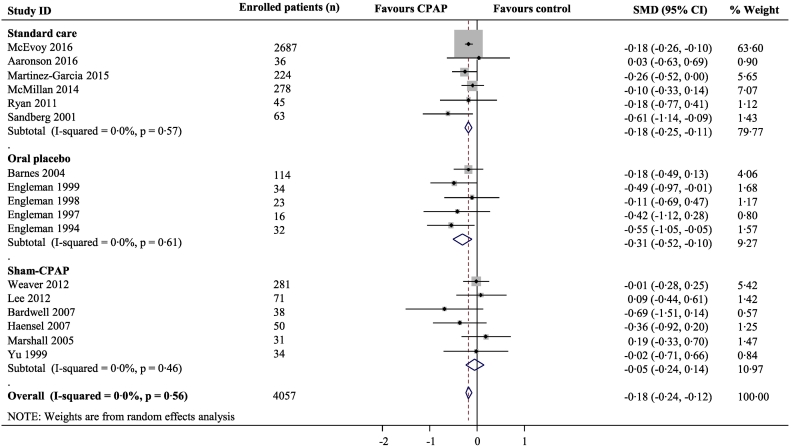
Fig. 2.
Meta-analysis of the effect of CPAP for depression symptoms
Pooled standardised mean difference (SMD) was calculated using a random effects model. Boxes are SMD and lines are 95% confidence interval (CI). The vertical solid line represents no difference between CPAP and control. Values to the left of the solid line favour CPAP benefit. Pooled SMDs and 95%CI are represented by the diamond shapes.
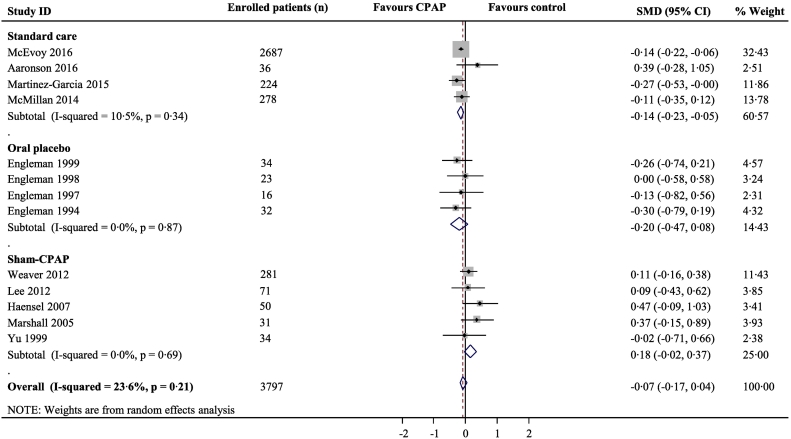
Fig. 3.
Meta-analysis of the effect of CPAP for anxiety symptoms
Pooled standardised mean difference (SMD) was calculated using a random effects model. Boxes are SMD and lines are 95% confidence interval (CI). The vertical solid line represents no difference between CPAP and control. Values to the left of the solid line favour CPAP benefit. Pooled SMD and 95%CI are represented by diamond shapes.
3.2. Systematic review and meta-analysis
Our systematic search identified 1624 articles, with full text review identifying 20 eligible studies (4255 participants) that evaluated changes in depression and/or anxiety symptoms in response to CPAP in OSA patients (Table 7, Fig. 5, Appendix). All 20 included trials reported on depression symptoms for CPAP, although only 17 (4057 participants) had the necessary numerical data for inclusion in a meta-analysis. The overall standardised mean difference for depression symptoms for CPAP versus control was − 0·18 (95%CI -0·24 to − 0·12) (Fig. 2). We identified 13 trials with outcomes related to anxiety symptoms for quantitative synthesis: the overall standardised mean difference for anxiety symptoms for CPAP versus control was − 0·07 (− 0·17 to 0·04) (Fig. 3). In subgroup meta-analysis stratified by the type of control group used, CPAP was superior to both standard care and oral placebo for depression symptoms, while anxiety symptoms were alleviated with CPAP only when compared to standard care. By contrast, there was no effect of CPAP treatment when compared to sham-CPAP treatment for either depression (Fig. 2) or anxiety (Fig. 3). Sensitivity analyses excluding the SAVE trial from meta-analyses showed similar results to the main analyses (Figs. 6–7, Appendix). The included trials were mostly of high quality (Fig. 8, Appendix) and there was low between-study heterogeneity (I2 values 0% and 23·6% for depression and anxiety studies respectively). There was no evidence of publication bias (Figs. 9–10 Appendix).
4. Discussion
Mood disorders are common in patients at high risk of CV disease, and depression in particular is associated with premature mortality and recurrent CV events [36], [37]. In the SAVE study of 2687 patients with established CV disease and OSA who were followed over several years, CPAP was found to significantly reduce the rates of clinically significant ‘caseness’ of depression but not of anxiety. The effect of CPAP occurred within several months of commencing the treatment, independently of improvements in daytime sleepiness, and was greater in those with pre-existing depression. Taken together with a systematic review of 20 randomised controlled studies, these results support a beneficial effect of CPAP treatment for the alleviation of depression symptoms.
The relationship between sleep disturbance and abnormal mood is strong and bidirectional [38], [39]. In OSA patients, there are a range of relevant pathophysiological mechanisms operating that include apnoea-induced sleep fragmentation, hypoxia, and daytime sleepiness, cerebral grey matter structural changes, and metabolic abnormalities, which may be reversed by CPAP treatment [3], [40], [41], [42], [43], [44]. This study defines a clear effect of CPAP treatment on depressive symptoms which acts independently of improvements in daytime sleepiness. Although analysis of depression symptoms as a continuous variable did not show any difference between CPAP and usual care groups at 72 months timepoint, this is likely due to small numbers of patients at this stage of the study. A detailed analysis of the individual components of the HADS-D also showed a consistent effect of CPAP treatment across all of the questions including those related to sleepiness, such as “feeling slowed down” and anhedonia-oriented symptoms. These effects remained after adjustment for the change in sleepiness (ESS) during follow-up. Sub-group analysis of the SAVE study also showed a greater effect of CPAP in those with prior depression, which is consistent with a prior systematic review [8]. As the NNT (i.e. 15) for CPAP to prevent depression is comparable to remission rates achieved for some established antidepressant drugs [45], our findings provide further support for the broader beneficial effects of CPAP in those with OSA, and especially those at high CV risk, where there is the potential for enhanced mood to improve long-term CV outcomes [29], [45].
Although improvements were observed in four (out of seven) individual components of the HADS-A subscale, the rate of anxiety caseness was not significantly lower among the CPAP treated patients at the end of study. The lack of significant difference in rates of anxiety caseness between the groups may be largely due to parallel improvements in symptoms in the usual care group, although an anxiety inducing effect of CPAP from, for example, from the mask application, may also be relevant. Another issue is a steady reduction in anxiety symptoms in both groups which might arise as a result of repeated testing and regular interactions with health care workers (i.e. the Hawthorne effect) [24].
Our systematic review and meta-analysis confirmed the efficacy of CPAP in reducing depression but not anxiety symptoms. The overall neutral effect of CPAP treatment on anxiety symptoms may be due to a slight positive, albeit non-significant effect of sham-CPAP as compared to therapeutic CPAP in several included trials. The reduction in depression symptoms from CPAP occurred when compared to placebo pill or usual care, but not when compared with sham-CPAP. Sham-CPAP is designed to provide flow of air via a face mask at very low sub-therapeutic pressure; the mask and pump being identical in appearance to true CPAP, with the exception that the mask has a larger hole for venting air. The use of sham-CPAP as a true control in CPAP studies has been controversial, and earlier studies have found small improvements in sleep and respiratory parameters as a result of its use [46], [47], [48]. A study on sham-CPAP reported small changes in OSA patients toward worse quality sleep but a reduced severity of sleep disordered breathing (complete airway obstruction [apneas] tending to be replaced by partial obstruction events [hypopneas]) and a reduced overnight burden of hypoxia [48]. It is possible, therefore, that sham-CPAP may obscure some of the effects of CPAP on depression and anxiety symptoms [49], and further research is warranted to better define the effects of sham-CPAP on mood.
Key strengths of these analyses are that they were derived from the SAVE study, the largest randomised evaluation of the effects of CPAP for OSA in CV patients who were well characterised and followed up in a range of clinical settings, thus providing high validity and generalisability of the findings. Moreover, the SAVE study allowed an evaluation of treatment effects on depression and anxiety symptoms over a substantially longer time period than has previously been undertaken, and incorporation of these data into a systematic review of other trials provided reliability and consistency of findings. However, there are some limitations, particularly in the use of screening tools to assess mood outcomes as opposed to gold standard, semi-structured, psychiatric interviews. Even so, self-reported depression and anxiety scores are more relevant to routine clinical practice (easy to administer, low cost), and are highly correlated with clinical diagnoses of depression and anxiety [26], [50]. Although we used recommended cut-point scores of eight on both HADS-A and HADS-D subscales to provide an optimum balance of sensitivity and specificity for abnormal mood in studies [26], screening instruments are prone to misdiagnosis and clinical uncertainty regarding appropriate clinical pathways for care. Another limitation is that we lacked information on psychotropic medicines which may confound the results, although patients with major mental illness would likely have avoided participating in such studies. Moreover, as we only included patients with coexisting moderate–severe OSA and CV disease, our results may not be generalisable to patients with mild OSA or broader OSA population without CV disease. For the systematic review, there are clear limitations to the various component trials, including the lack of blinding of patients or their outcome assessors, short duration of follow-up, and variable adherence to CPAP. Finally, a few studies were excluded due to a lack of numerical data for statistical synthesis [18], [22], [51].
5. Conclusion
In summary, our detailed analysis of the SAVE study has shown that CPAP for moderate–severe OSA in patients with CV disease has broader benefits in terms of preventing depression caseness, independent of improved sleepiness. Our additional systematic review provides further support of the treatment effect of CPAP for depression, but not for anxiety symptoms.
Funding
National Health and Medical Research Council of Australia, Respironics Sleep and Respiratory Research Foundation and Philips Respironics.
Author contributions
Conception: DZ, RDM, and CSA.
Data acquisition: DZ, YX, SY, LFD, GLF, MT, OM, QO, RC, ZL, XZ, YL, NM, RDM, and CSA.
Statistical analysis: DZ, RJW, QL, and MW.
Drafting of the initial manuscript: DZ, RDM, and CSA.
Interpretation of data and critical revision of the manuscript for important intellectual content: DZ, YX, SY, MLH, RJW, QL, MW, KAL, AR, LFD, GLF, XW, WWQ, MT, OM, QO, RC, ZL, XZ, YL, NM, SM, RDM, and CSA.
The leading (DZ) and corresponding (CSA) authors confirm having full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interest
MLH is funded by a National Heart Foundation Future Leader Fellowship. MW is an NHMRC of Australia Principal Research Fellow (1080206) and received consulting fees from Amgen and Kirin. NM received grants from Oventus Pty Ltd., Nyxoah Pty Ltd. and Zelda therapeutics. RDM has received grants and nonfinancial support from Philips Respironics, nonfinancial support from ResMed, grants from the National Health and Medical Research Council of Australia, and grants from Fisher & Paykel, during the conduct of the study; nonfinancial support from Air Liquide; and speaker fees from ResMed. CSA holds a NHMRC Senior Principal Research Fellowship and has received fees Amgen and Boehringer Ingelheim for participating in advisory panels, from Takeda China for speaking at seminars. DZ, YX, SY, RJW, QL, KAL, AR, LFD, GLF, XW, WWQ, MT, OM, QO, RC, ZL, XZ, YL, NM and SM have nothing to disclose.
Acknowledgments
Acknowledgements
The SAVE trial was supported by project grants (1006501 [2011–2015] and 1060078 [2014–2016]) from the National Health and Medical Research Council (NHMRC) of Australia and by Respironics Sleep and Respiratory Research Foundation and Philips Respironics. Supplementary trial funding was provided by Fisher & Paykel Healthcare, the Australasian Sleep Trials Network (enabling grant 343020 from the NHMRC), the Spanish Respiratory Society (grant 105-2011 to Drs. Barbe and Mediano), and Fondo de Investigaciones Sanitarias (grant 13/02053 to Drs. Barbe and Mediano). In-kind donations were provided by Respironics for CPAP equipment and by ResMed for sleep apnoea diagnostic devices. All funders had no involvement in the study design, in the collection, analysis, and interpretation of data; in writing of the report; and in the decision to submit the paper for publication.
Data availability
Deidentified data collected for the study including participant data and a data dictionary will be made available to other researchers in Jan 2020.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.05.012.
Appendix A. Supplementary data
Supplementary material
References
- 1.Ohayon M.M. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64:1195–1200. doi: 10.4088/jcp.v64n1009. [quiz, 1274-6] [DOI] [PubMed] [Google Scholar]; Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry 2003; 64: 1195-200; quiz, 1274-6. [DOI] [PubMed]
- 2.Sharafkhaneh A., Giray N., Richardson P., Young T., Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–1411. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]; Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep 2005; 28: 1405–11. [DOI] [PubMed]
- 3.Wheaton A.G., Perry G.S., Chapman D.P., Croft J.B. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005–2008. Sleep. 2012;35:461–467. doi: 10.5665/sleep.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005-2008. Sleep 2012; 35: 461–67. doi:10.5665/sleep.1724. [DOI] [PMC free article] [PubMed]
- 4.Harris M., Glozier N., Ratnavadivel R., Grunstein R.R. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13:437–444. doi: 10.1016/j.smrv.2009.04.001. [DOI] [PubMed] [Google Scholar]; Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev 2009; 13: 437–44. doi:10.1016/j.smrv.2009.04.001. [DOI] [PubMed]
- 5.Saunamäki T., Jehkonen M. Depression and anxiety in obstructive sleep apnea syndrome: a review. Acta Neurol Scand. 2007;116:277–288. doi: 10.1111/j.1600-0404.2007.00901.x. [DOI] [PubMed] [Google Scholar]; Saunamäki T, Jehkonen M. Depression and anxiety in obstructive sleep apnea syndrome: a review. Acta Neurol Scand 2007; 116: 277–88. doi:10.1111/j.1600-0404.2007.00901.x. [DOI] [PubMed]
- 6.Gupta M.A., Simpson F.C., Lyons D.C.A. The effect of treating obstructive sleep apnea with positive airway pressure on depression and other subjective symptoms: a systematic review and meta-analysis. Sleep Med Rev. 2016;28:55–68. doi: 10.1016/j.smrv.2015.07.002. [DOI] [PubMed] [Google Scholar]; Gupta MA, Simpson FC, Lyons DCA. The effect of treating obstructive sleep apnea with positive airway pressure on depression and other subjective symptoms: A systematic review and meta-analysis. Sleep Med Rev 2016; 28: 55–68. doi:10.1016/j.smrv.2015.07.002. [DOI] [PubMed]
- 7.Kendzerska T., Gershon A.S., Hawker G.A., Tomlinson G.A., Leung R.S. Obstructive sleep apnoea is not a risk factor for incident hospitalised depression: a historical cohort study. Eur Respir J. 2017;49 doi: 10.1183/13993003.01361-2016. [DOI] [PubMed] [Google Scholar]; Kendzerska T, Gershon AS, Hawker GA, Tomlinson GA, Leung RS. Obstructive sleep apnoea is not a risk factor for incident hospitalised depression: a historical cohort study. Eur Respir J 2017; 49. doi:10.1183/13993003.01361-2016. [DOI] [PubMed]
- 8.Povitz M., Bolo C.E., Heitman S.J., Tsai W.H., Wang J., James M.T. Effect of treatment of obstructive sleep apnea on depressive symptoms: systematic review and meta-analysis. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001762. [DOI] [PMC free article] [PubMed] [Google Scholar]; Povitz M, Bolo CE, Heitman SJ, Tsai WH, Wang J, James MT. Effect of treatment of obstructive sleep apnea on depressive symptoms: systematic review and meta-analysis. PLoS Med 2014; 11: e1001762. doi:10.1371/journal.pmed.1001762. [DOI] [PMC free article] [PubMed]
- 9.Patil S.P., Ayappa I.A., Caples S.M., Kimoff R.J., Patel S.R., Harrod C.G. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15:301–334. doi: 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]; Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med 2019; 15: 301–34. doi:10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed]
- 10.Gupta M.A., Simpson F.C. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med. 2015;11:165–175. doi: 10.5664/jcsm.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med 2015; 11: 165–75. doi:10.5664/jcsm.4466. [DOI] [PMC free article] [PubMed]
- 11.Aaronson J.A., Hofman W.F., van Bennekom C.A.M. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2016;12:533–541. doi: 10.5664/jcsm.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aaronson JA, Hofman WF, van Bennekom CAM, et al. Effects of Continuous Positive Airway Pressure on Cognitive and Functional Outcome of Stroke Patients with Obstructive Sleep Apnea: A Randomized Controlled Trial. J Clin Sleep Med 2016; 12: 533–41. doi:10.5664/jcsm.5684. [DOI] [PMC free article] [PubMed]
- 12.Bardwell W.A., Norman D., Ancoli-Israel S. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med. 2007;5:21–38. doi: 10.1207/s15402010bsm0501_2. [DOI] [PubMed] [Google Scholar]; Bardwell WA, Norman D, Ancoli-Israel S, et al. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med 2007; 5: 21–38. doi:10.1080/15402000709336724. [DOI] [PubMed]
- 13.Engleman H.M., Martin S.E., Deary I.J., Douglas N.J. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343:572–575. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]; Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994; 343: 572–75. [DOI] [PubMed]
- 14.Engleman H.M., Martin S.E., Deary I.J., Douglas N.J. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:114–119. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax 1997; 52: 114–19. [DOI] [PMC free article] [PubMed]
- 15.Engleman H.M., Martin S.E., Kingshott R.N., Mackay T.W., Deary I.J., Douglas N.J. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341–345. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]; Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax 1998; 53: 341–45. [DOI] [PMC free article] [PubMed]
- 16.Engleman H.M., Kingshott R.N., Wraith P.K., Mackay T.W., Deary I.J., Douglas N.J. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care. 1999;159:461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]; Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care 1999; 159: 461–67. doi:10.1164/ajrccm.159.2.9803121. [DOI] [PubMed]
- 17.Haensel A., Norman D., Natarajan L., Bardwell W.A., Ancoli-Israel S., Dimsdale J.E. Effect of a 2 week CPAP treatment on mood states in patients with obstructive sleep apnea: a double-blind trial. Sleep Breath. 2007;11:239–244. doi: 10.1007/s11325-007-0115-0. [DOI] [PubMed] [Google Scholar]; Haensel A, Norman D, Natarajan L, Bardwell WA, Ancoli-Israel S, Dimsdale JE. Effect of a 2 week CPAP treatment on mood states in patients with obstructive sleep apnea: a double-blind trial. Sleep Breath 2007; 11: 239–44. doi:10.1007/s11325-007-0115-0. [DOI] [PubMed]
- 18.Henke K.G., Grady J.J., Kuna S.T. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea-hypopnea syndrome. A randomized, placebo-controlled trial. Am J Respir Crit Care. 2001;163:911–917. doi: 10.1164/ajrccm.163.4.9910025. [DOI] [PubMed] [Google Scholar]; Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea-hypopnea syndrome. A randomized, placebo-controlled trial. Am J Respir Crit Care 2001; 163: 911–17. doi:10.1164/ajrccm.163.4.9910025. [DOI] [PubMed]
- 19.Lee I.-S., Bardwell W., Ancoli-Israel S., Loredo J.S., Dimsdale J.E. Effect of three weeks of continuous positive airway pressure treatment on mood in patients with obstructive sleep apnoea: a randomized placebo-controlled study. Sleep Med. 2012;13:161–166. doi: 10.1016/j.sleep.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee I-S, Bardwell W, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effect of three weeks of continuous positive airway pressure treatment on mood in patients with obstructive sleep apnoea: a randomized placebo-controlled study. Sleep Med 2012; 13: 161–66. doi:10.1016/j.sleep.2011.09.005. [DOI] [PMC free article] [PubMed]
- 20.Marshall N.S., Neill A.M., Campbell A.J., Sheppard D.S. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax. 2005;60:427–432. doi: 10.1136/thx.2004.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]; Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax 2005; 60: 427–32. doi:10.1136/thx.2004.032078. [DOI] [PMC free article] [PubMed]
- 21.Ryan C.M., Bayley M., Green R., Murray B.J., Bradley T.D. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–1067. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]; Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke 2011; 42: 1062–67. doi:10.1161/STROKEAHA.110.597468. [DOI] [PubMed]
- 22.Redline S., Adams N., Strauss M.E., Roebuck T., Winters M., Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care. 1998;157:858–865. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]; Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care 1998; 157: 858–65. doi:10.1164/ajrccm.157.3.9709042. [DOI] [PubMed]
- 23.Sandberg O., Franklin K.A., Bucht G., Gustafson Y. Sleep apnea, delirium, depressed mood, cognition, and ADL ability after stroke. J Am Geriatr Society. 2001;49:391–397. doi: 10.1046/j.1532-5415.2001.49081.x. [DOI] [PubMed] [Google Scholar]; Sandberg O, Franklin KA, Bucht G, Gustafson Y. Sleep apnea, delirium, depressed mood, cognition, and ADL ability after stroke. J Am Geriatr Society 2001; 49: 391–97. [DOI] [PubMed]
- 24.Yu B.H., Ancoli-Israel S., Dimsdale J.E. Effect of CPAP treatment on mood states in patients with sleep apnea. J Psychiatr Res. 1999;33:427–432. doi: 10.1016/s0022-3956(99)00020-5. [DOI] [PubMed] [Google Scholar]; Yu BH, Ancoli-Israel S, Dimsdale JE. Effect of CPAP treatment on mood states in patients with sleep apnea. J Psychiatr Res 1999; 33: 427–32. [DOI] [PubMed]
- 25.McEvoy R.D., Antic N.A., Heeley E. CPAP for prevention of cardiovascular events in obstructive sleep apnea. New Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]; McEvoy RD, Antic NA, Heeley E, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. New Engl J Med 2016; 375: 919–31. doi:10.1056/NEJMoa1606599. [DOI] [PubMed]
- 26.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the hospital anxiety and depression scale. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]; Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. J Psychosom Res 2002; 52: 69–77. doi:10.1016/S0022-3999(01)00296-3. [DOI] [PubMed]
- 27.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]; Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–70. [DOI] [PubMed]
- 28.Cook R.J., Sackett D.L. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995; 310: 452–54. doi:10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed]
- 29.Yu J., Zhou Z., McEvoy R.D. Association of Positive Airway Pressure with Cardiovascular Events and Death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156–166. doi: 10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yu J, Zhou Z, McEvoy RD, et al. Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis. JAMA 2017; 318: 156–66. doi:10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed]
- 30.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]; Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed]
- 31.Beck A.T., Steer R.A., Ball R., Ranieri W. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]; Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996; 67: 588–97. doi:10.1207/s15327752jpa6703_13. [DOI] [PubMed]
- 32.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]; Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–89. [DOI] [PubMed]
- 33.Nyenhuis D.L., Yamamoto C., Luchetta T., Terrien A., Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55:79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]; Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol 1999; 55: 79–86. [DOI] [PubMed]
- 34.Yesavage J.A., Brink T.L., Rose T.L. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]; Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17: 37–49. [DOI] [PubMed]
- 35.The Cochrane Collaboration Assessing risk of Bias in included studies. https://methods.cochrane.org/bias/assessing-risk-bias-included-studies [accessed Jan 2018]; The Cochrane Collaboration. Assessing Risk of Bias in Included Studies. https://methods.cochrane.org/bias/assessing-risk-bias-included-studies (accessed Jan 2018).
- 36.Lespérance F., Frasure-Smith N., Talajic M., Bourassa M.G. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]; Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002; 105: 1049–53. [DOI] [PubMed]
- 37.Drudi L.M., Ades M., Turkdogan S. Association of Depression with mortality in older adults undergoing transcatheter or surgical aortic valve replacement. JAMA Cardiol. 2018;3:191–197. doi: 10.1001/jamacardio.2017.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Drudi LM, Ades M, Turkdogan S, et al. Association of Depression With Mortality in Older Adults Undergoing Transcatheter or Surgical Aortic Valve Replacement. JAMA cardiology 2018; 3: 191–97. doi:10.1001/jamacardio.2017.5064. [DOI] [PMC free article] [PubMed]
- 38.Lyall L.M., Wyse C.A., Graham N. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry. 2018;5:507–514. doi: 10.1016/S2215-0366(18)30139-1. [DOI] [PubMed] [Google Scholar]; Lyall LM, Wyse CA, Graham N, et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry 2018; 5: 507–14. doi:10.1016/S2215-0366(18)30139-1. [DOI] [PubMed]
- 39.Kahn M., Sheppes G., Sadeh A. Sleep and emotions: bidirectional links and underlying mechanisms. Int J Psychophysiol. 2013;89:218–228. doi: 10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed] [Google Scholar]; Kahn M, Sheppes G, Sadeh A. Sleep and emotions: bidirectional links and underlying mechanisms. Int J Psychophysiol 2013; 89: 218–28. doi:10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed]
- 40.Canessa N., Castronovo V., Cappa S.F. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care. 2011;183:1419–1426. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]; Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care 2011; 183: 1419–26. doi:10.1164/rccm.201005-0693OC. [DOI] [PubMed]
- 41.Martínez-Cerón E., Barquiel B., Bezos A.-M. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care. 2016;194:476–485. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]; Martínez-Cerón E, Barquiel B, Bezos A-M, et al. Effect of Continuous Positive Airway Pressure on Glycemic Control in Patients with Obstructive Sleep Apnea and Type 2 Diabetes. A Randomized Clinical Trial. Am J Respir Crit Care 2016; 194: 476–85. doi:10.1164/rccm.201510-1942OC. [DOI] [PubMed]
- 42.Martínez-Ceron E., Fernández-Navarro I., Garcia-Rio F. Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med Rev. 2016;25:121–130. doi: 10.1016/j.smrv.2015.03.002. [DOI] [PubMed] [Google Scholar]; Martínez-Ceron E, Fernández-Navarro I, Garcia-Rio F. Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med Rev 2016; 25: 121–30. doi:10.1016/j.smrv.2015.03.002. [DOI] [PubMed]
- 43.Feng Y., Zhang Z., Dong Z.-Z. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005. doi: 10.1038/npjpcrm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Feng Y, Zhang Z, Dong Z-Z. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med 2015; 25: 15005. doi:10.1038/npjpcrm.2015.5. [DOI] [PMC free article] [PubMed]
- 44.Iftikhar I.H., Hoyos C.M., Phillips C.L., Magalang U.J. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475–485. doi: 10.5664/jcsm.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]; Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analyses of the Association of Sleep Apnea with Insulin Resistance, and the Effects of CPAP on HOMA-IR, Adiponectin, and Visceral Adipose Fat. J Clin Sleep Med 2015; 11: 475–85. doi:10.5664/jcsm.4610. [DOI] [PMC free article] [PubMed]
- 45.Cipriani A., Furukawa T.A., Salanti G. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018; 391: 1357–66. doi:10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed]
- 46.Zhao Y.Y., Wang R., Gleason K.J. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: best apnea interventions for research (BestAIR) trial. Sleep. 2017;40 doi: 10.1093/sleep/zsx040. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhao YY, Wang R, Gleason KJ, et al. Effect of Continuous Positive Airway Pressure Treatment on Health-Related Quality of Life and Sleepiness in High Cardiovascular Risk Individuals With Sleep Apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017; 40. doi:10.1093/sleep/zsx040. [DOI] [PMC free article] [PubMed]
- 47.Montserrat J.M., Ferrer M., Hernandez L. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care. 2001;164:608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]; Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care 2001; 164: 608–13. doi:10.1164/ajrccm.164.4.2006034. [DOI] [PubMed]
- 48.Rodway G.W., Weaver T.E., Mancini C. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33:260–266. doi: 10.1093/sleep/33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep 2010; 33: 260–66. [DOI] [PMC free article] [PubMed]
- 49.Quan S.F., Chan C.S., Dement W.C. The association between obstructive sleep apnea and neurocognitive performance—the apnea positive pressure long-term efficacy study (APPLES) Sleep. 2011;34:303–314B. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]; Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance—the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2011; 34: 303-314B. [DOI] [PMC free article] [PubMed]
- 50.Stafford L., Berk M., Jackson H.J. Validity of the hospital anxiety and depression scale and patient health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry. 2007;29:417–424. doi: 10.1016/j.genhosppsych.2007.06.005. [DOI] [PubMed] [Google Scholar]; Stafford L, Berk M, Jackson HJ. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry 2007; 29: 417–24. doi:10.1016/j.genhosppsych.2007.06.005. [DOI] [PubMed]
- 51.Barnes M., McEvoy R.D., Banks S. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care. 2004;170:656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]; Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care 2004; 170: 656–64. doi:10.1164/rccm.200311-1571OC. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Deidentified data collected for the study including participant data and a data dictionary will be made available to other researchers in Jan 2020.