Figure 4. Known Non-Canonical Roles of EZH2.
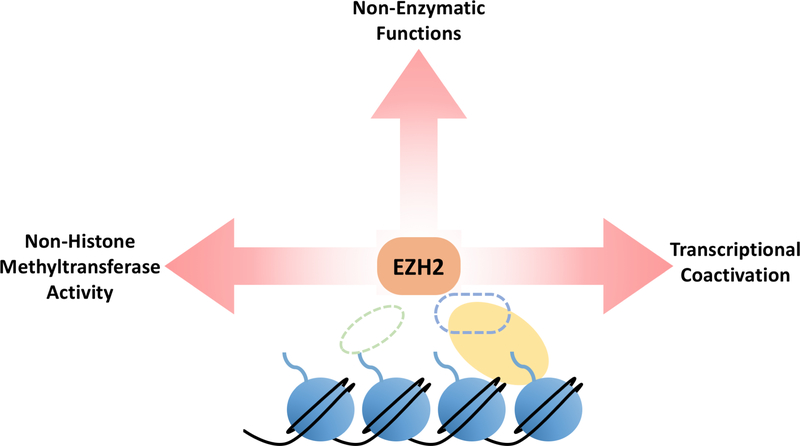
While SUZ12 or EED are mutated in approximately 85% of MPNSTs, EZH2 loss of function has not been observed. Overexpression of EZH2 has been reported in MPNSTs. EZH2 inhibition and depletion have also proven efficacious in slowing MPNST growth in preclinical models despite PRC2 already being genetically inactivated. This suggests that EZH2 may assume pathogenic functions in MPNST in the absence of other PRC2 core components. Non-canonical, pathogenic roles for EZH2 have been demonstrated in numerous other contexts. For example, overexpression of catalytically inactive EZH2 in natural killer/T-cell lymphoma conferred a growth advantage to these cells. Phosphorylated EZH2 has been shown to contribute to glioblastoma, breast cancer, and prostate cancer tumorigenicity through divergent mechanisms. The efficacy of EZH2 inhibition and depletion in slowing MPNST growth in preclinical models, as well as the clearly established non-canonical roles of EZH2 in other contexts represent an understudied avenue for potential MPNST therapies.