Table 4.
4(1H)-Quinolones Substituted in 6-Position with the Piperazine Moiety
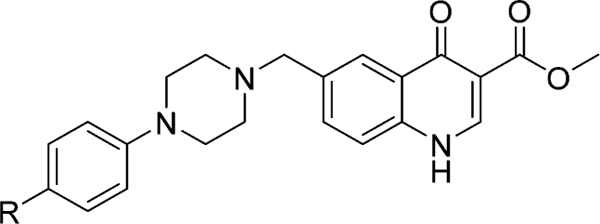 | ||||||
|---|---|---|---|---|---|---|
| compd | R | EC50 W2 (nM)a |
EC50 TM90- C2B (nM) |
RIb | EC50
Pb
(nM) |
EC50 J774 (μM) |
| 8af | −H | 150 | >2000 | >13 | 91 | >20 |
| 8ag | −OCH3 | 160 | >2000 | >12 | >100 | >20 |
| 8ah | −F | 110 | >2000 | >18 | c | >20 |
| 8ai | −CF3 | 45 | >2000 | >44 | 85 | >20 |
Chloroquine (CQ), atovaquone (ATO), and dihydroartemisinin (DHA) are internal controls for each in vitro assay: CQ, 0.42 μM W2, 0.23 μM TM90-C2B, and 47.2 μM J774; ATO, 1.4 nM W2, 18 μM TM90-C2B, and 28 μM J774; DHA, 5.5 nM W2, 5.9 nM TM90-C2B, and 1.5 μM J774.
RI = TM90-C2B/W2.
Not determined.
