Abstract
BACKGROUND:
After the emergence of new influenza viruses, the morbidity and mortality of viral pneumonia have received a great attention.
OBJECTIVES:
The objective of this study is to describe the epidemiologic, clinical and laboratory changes, and outcomes of viral pneumonia caused by influenza and the Middle East respiratory syndrome-coronavirus (MERS-CoV) infections.
METHODS:
In a retrospective cohort study, the medical records of all patients diagnosed with viral pneumonia at Prince Sultan Military Medical City, Riyadh, Saudi Arabia, during the period from January 2012 to December 2015 were screened. Cases who were > 18 years old and were confirmed by a respiratory viral panel to have viral pneumonia either MERS-CoV or influenza viruses were included in the analysis. Sociodemographic, clinical, laboratory, and outcome data were extracted from patients' medical files. The data were analyzed descriptively and inferentially to identify the predictors of poor outcome.
RESULTS:
A total of 448 patients with confirmed viral pneumonia were included, of those, 216 (48.2%) were caused by influenza A (non H1N1)/influenza B, 150 (33.5%) by H1N1, and 82 (18.3%) by MERS-CoV. The majority of patients presented with fever (82%), shortness of breath (64%), and flu-like symptoms (54.9%), particularly in MERS-CoV infected cases (92%). The peak incidence of viral pneumonia was in early spring and autumn. The mortality rate was 13.8%, and it was significantly higher among MERS-CoV cases. The predictors of death were age > 65 years, male gender, and associated comorbidities particularly diabetes mellitus, hypertension, and chronic kidney diseases. The number of comorbid illnesses was directly related to the increase in mortality in this group of patients.
CONCLUSION:
Viral pneumonia caused by influenza and MERS-CoV carries a high mortality rate, particularly among MERS-CoV infected cases. Old age, male gender, and comorbid illnesses are predictors of poor outcome. Routine testing for newly emergent viruses is warranted for adults who have been hospitalized with pneumonia.
Keywords: Viral pneumonia, Middle East respiratory syndrome-coronavirus, influenza, H1N1
Pneumonia is a leading cause of hospitalization worldwide and carries significant morbidity and mortality that differ according to the underlying etiology.[1] In general, viral pneumonia is considered less severe compared to bacterial pneumonia.[2] In the past decade, however, the mortality related to viral pneumonia has substantially increased because of the emergence of the new respiratory viruses such as influenza A H1N1 and the Middle East respiratory syndrome-coronavirus (MERS-CoV).[3] Moreover, high mortality was reported in previously healthy middle-aged patients during influenza A H1N1 pandemic infection in 2009.[4] Reports from the southern hemisphere countries suggested that the hospitalization rates secondary to influenza A H1N1 ranged from 23.6% to 30.6%; among them, 11.7% to 18.5% were admitted to the Intensive Care Units (ICUs) with mortality rate ranged from 16% to 41%.[5] Noteworthy, according to the last update from the center for diseases control (CDC) on February 2014, pneumonia and influenza were the cause of death in 8.4% of all deaths in the United States, of which 34% occurred in persons aged ≥65 years, 62% in persons aged 25–64 years, and 4% in persons younger than 24 years old.[6]
Data from Saudi Arabia have suggested comparable results to other countries, such as Europe, North America, and Japan regarding the young age predilection of influenza A H1N1.[7,8] A Saudi report on the first 100 cases of pandemic influenza A H1N1 described that most of the patients were younger than 40 years and the hospitalization rate among them was as high as 10%. Most of these patients had underlying conditions such as cardiovascular disease, respiratory diseases, autoimmune disorders, obesity, diabetes, cancer, or pregnancy.[9] The clinical presentation varied from asymptomatic cases to viral pneumonia resulting in respiratory failure, acute respiratory distress syndrome, multiorgan failure, or death. Interestingly, gastrointestinal symptoms including diarrhea and vomiting accounted for up to 50% of clinical presentation.[10]
As of July 2017, the World Health Organization (WHO) had received reports from 27 countries of 2040 cases of laboratory-confirmed MERS and at least 677 related deaths representing case fatality of 35%.[11] The course of the disease has been described to vary from asymptomatic viral illness to dramatically fatal respiratory failure and ARDS. Gastrointestinal and renal involvement have also been reported in one-third and one-half of the cases, respectively.[12] In Saudi Arabia, MERS-CoV virus was first reported in 2012, and between September 1, 2012, and June 15, 2013, there have been 47 laboratory-confirmed cases (46 adults and one child) with a fatality rate of 60%[13] Assiri et al., had reported the epidemiological characteristics of the MERS-CoV in Saudi Arabia either as a community cases running in clusters within the families or among healthcare workers with or without direct camel contacts.[13] During June–August, 2015, a second MERS-CoV outbreak occurred with a total of 130 MERS-CoV cases detected. Overall, 96 patients (74%) required hospitalization, of them 63 (66%) required intensive care management, and the fatality rate was 53%.[14]
The available studies describing the clinical features and the outcome of influenza H1N1 and MERS-CoV are limited by small sample size or a short follow-up duration.
The present study aimed to describe the clinical and laboratory characteristic of patients presented with viral pneumonia secondary to influenza A/H1N1, influenza A/non-H1N1, influenza B, and MERS-CoV to Prince Sultan Military Medical City (PSMMC) and their disease outcomes over a 4-year period.
Methods
A retrospective analysis of a cohort of viral pneumonia cases presented to PSMMC between January 2012 and December 2015 was done. PSMMC is a tertiary health care center, with more than 1500 beds, in Riyadh, the capital city of Saudi Arabia. The patients were included if they had positive respiratory virus panel for MERS-CoV or influenza and pneumonia defined as acute respiratory illness and compatible chest X-ray findings at presentation or within 48 h of admission.
The study included adult patients over 18 years of age whether managed as in- or out-patients.
Data were collected from patients' hospital records and microbiology department. The specimens of both upper (nasopharyngeal, oropharyngeal swabs, and sputum) and lower respiratory tract (tracheal aspirates and bronchoalveolar lavage) were tested by molecular testing GenXpert (Cepheid) for the respiratory viruses including influenza A/H1N1, Influenza A/non-H1N1, influenza B and MERS-CoV.
Demographic data, symptoms, and signs at presentation, biochemical and radiological results, site of care, hospital stay, and overall outcome were also recorded. Descriptive epidemiology was performed to depict the epidemic curve (number of cases vs. time of diagnosis) and to assess the case fatality rate according to age group. Approval was granted from PSMMC Institutional Research Board (IRB).
The statistical analysis was performed using SPSS software, version 22.0, for Windows (SPSS, Inc., Chicago, IL, USA). Categorical variables were presented as frequencies and percentages while continuous variables were presented as mean and standard deviation. Comparison patients' baseline and clinical characteristics of viral pneumonia groups were done using Chi-square test and ANOVA analysis as appropriate. Univariate logistic regression was also performed to examine factors that are associated with mortality with the level of statistical significance being set at <0.05.
Results
A total of 448 confirmed viral pneumonia patients were included in the analysis. Of those, 216 (48.2%) cases were infected with influenza A non-H1N1/influenza B, 150 (33.5%) with H1N1, and 82 (18.3%) with MERS-CoV. The baseline characteristics of the studied patients are presented in Table 1. The mean age of the studied cohort was 55.7 ± 22.1 years, and it was significantly higher among cases infected with MERS CoV (61.6 ± 21.0) and Influenza A non-H1N1/influenza B (56.5 ± 23.1) (P = 0.002). Two hundred thirty-five patients (52.5%) were male, and 35 (16.4%) of the female patients were pregnant at the time of infection. Cough and fever were the most prevalent symptoms (84.4 and 82% respectively). Flue-like illness was common among MERS-CoV (92%) and H1N1 (63.3%) infected cases. Diabetes mellitus and hypertension were found in nearly half of the studied cases (47.1% for DM and 52.3% for hypertension), and they were more prevalent in cases with H1N1 and MERS-CoV.
Table 1.
Baseline patient’s characteristics
| Characteristics* | All cases (n=448) | Influenza A (non-H1N1)/B (n=216) | H1N1 (n=150) | MERS-CoV (n=82) | P |
|---|---|---|---|---|---|
| Age (years) | 55.7±22.1 | 56.5±23.1 | 51.4±20.2 | 61.6±21.0 | 0.002 |
| Sex | |||||
| Male | 235 (52.5) | 105 (48.6) | 77 (51.3) | 53 (64.6) | 0.04 |
| Female | 213 (47.5) | 111 (51.4) | 73 (48.7) | 29 (35.4) | |
| Pregnancy** | |||||
| Yes | 35 (16.4) | 19 (17.1) | 14 (19.1) | 2 (6.8) | 0.16 |
| No | 178 (83.6) | 92 (82.9) | 59 (80.9) | 27 (93.2) | |
| Year of infection | |||||
| 2012 | 77 (17.2) | 40 (18.51) | 36 (24) | 1 (1.21) | <0.0001 |
| 2013 | 78 (17.4) | 57 (26.3) | 7 (4.66) | 14 (17.07) | |
| 2014 | 114 (25.4) | 54 (25) | 24 (16.0) | 36 (43.90) | |
| 2015 | 179 (40.0) | 65 (30) | 83 (55.3) | 31 (37.80) | |
| Symptoms | |||||
| Cough | 357 (84.4) | 174 (80.5) | 129 (59.7) | 54 (65.9) | 0.001 |
| Hypoxia | 63 (15.1) | 22 (10.2) | 24 (16) | 17 (20.7) | 0.03 |
| GI | 48 (13.5) | 28 (12.9) | 14 (9.3) | 6 (7.3) | 0.29 |
| Neurological | 37 (10.4) | 22 (10.2) | 11 (7.3) | 4 (4.9) | 0.29 |
| Fatigue | 25 (7.0) | 11 (5.1) | 7 (4.7) | 7 (8.5) | 0.31 |
| Flu symptoms | 246 (54.9) | 116 (53.7) | 95 (63.3) | 76 (92.0) | 0.02 |
| Chest pain | 65 (15.4) | 29 (13.4) | 24 (16.0) | 12 (14.6) | 0.80 |
| SOB | 287 (64.0) | 132 (61.1) | 107 (71.3) | 48 (58.5) | 0.14 |
| Fever | 369 (82.0) | 179 (82.9) | 131 (87.3) | 59 (71.9) | 0.04 |
| Associated diseases | |||||
| DM | 194 (47.1) | 93 (43.1) | 56 (37.3) | 5 (54.9) | 0.01 |
| Hypothyroidism | 18 (7.0) | 10 (4.6) | 5 (3.3) | 3 (3.7) | 0.60 |
| HTN | 214 (52.3) | 101 (46.8) | 65 (43.3) | 48 (58.5) | 0.01 |
| Dialysis | 36 (8.8) | 13 (6.0) | 11 (7.3) | 12 (14.6) | 0.02 |
| CKD | 64 (15.9) | 21 (9.7) | 25 (16.7) | 18 (21.9) | 0.01 |
| Chronic lung disease | 86 (19.2) | 53 (24.5) | 26 (12.0) | 7 (8.5) | 0.01 |
| Bed bound | 46 (11.2) | 23 (10.6) | 8 (3.7) | 15 (18.3) | 0.01 |
| HF | 44 (10.9) | 22 (10.2) | 10 (6.7) | 12 (14.6) | 0.07 |
| Transplant | 10 (2.4) | 4 (1.9) | 5 (3.3) | 1 (1.2) | 0.57 |
| Stroke | 32 (13.1) | 17 (7.9) | 7 (4.7) | 8 (9.8) | 0.06 |
| Liver disease | 15 (6.1) | 7 (3.2) | 6 (4.0) | 2 (2.4) | 0.48 |
| Cancer | 18 (4.4) | 10 (4.6) | 4 (2.7) | 4 (4.9) | 0.78 |
*Data are presented by mean±SD or by n (%), **Data presented for female cases (n=213). GI=Gastrointestinal, SOB=Shortness of breath, DM=Diabetes mellitus, HTN=Hypertension, CKD=Chronic kidney disease, SD=Standard deviation, HF=Heart failure, MERS-CoV=Middle east respiratory syndrome-corona virus
The incidence of viral pneumonia was highest between October and December followed by January–March [Figure 1]. The frequency of the causative virus has varied over the study years where the predominant virus was influenza A non-H1N1 in 2014, H1N1 in 2015, and MERS-Cov in 2014 and 2015.
Figure 1.
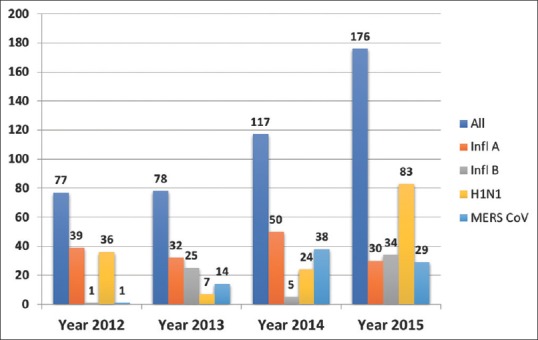
Epidemic curve (frequency of cases to time) of the studied viral pneumonia cases by year of diagnosis and their associated influenza and the Middle East respiratory syndrome-coronavirus infection, Riyadh, KSA, from June, 2012 to October, 2015 (total: 448 cases)
About two-thirds of the patients were treated in the general ward while 57 patients (13.3%) required admission to ICU; of them, 23 (40.3%) were cases associated with MERS-CoV. The distribution of CURB scores varied significantly based on the causative virus. A large proportion of patients had bilateral lung findings on chest X-ray, which was more frequent among MERS-CoV (94.1%) and H1N1 (86.7%) cases, although the difference was not statistically significant (P = 0.25) [Table 2].
Table 2.
Clinical indicators of viral pneumonia cases according to causative virus
| All cases (n=448) | Influenza A/B (n=216) | H1N1 (n=150) | MERS-CoV (n=82) | P | |
|---|---|---|---|---|---|
| Site of care* | |||||
| Ward | 282 (62.9) | 134 (62.0) | 101 (67.3) | 47 (57.3) | <0.0001 |
| ER | 72 (16.1) | 43 (19.9) | 27 (18.0) | 2 (2.4) | |
| ICU | 57 (12.7) | 19 (8.8) | 15 (10.0) | 23 (28.0) | |
| OPD | 16 (3.6) | 10 (4.6) | 4 (2.7) | 2 (2.4) | |
| Not reported | 21 (4.7) | 10 (4.6) | 3 (2) | 8 (9.8) | |
| CURB65 | |||||
| 0 | 190 (42.4) | 92 (42.6) | 78 (52.0) | 20 (24.4) | <0.0001 |
| 1 | 117 (26.1) | 61 (28.2) | 38 (25.3) | 18 (21.9) | |
| 2 | 60 (13.4) | 33 (15.3) | 11 (7.3) | 14 (17.1) | |
| 3 | 36 (8.0) | 13 (6.0) | 11 (7.3) | 12 (14.6) | |
| 4 | 22 (4.9) | 7 (3.2) | 5 (3.3) | 10 (12.2) | |
| 5 | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (1.2) | |
| Not reported | 22 (4.9) | 10 (4.6) | 7 (4.7) | 7 (8.5) | |
| CXR finding | |||||
| Unilateral infiltrate | 41 (9.2) | 21 (9.7) | 14 (9.3) | 4 (4.9) | 0.25 |
| Bilateral bilateral infiltrate | 262 (58.5) | 108 (50.0) | 91 (60.7) | 63 (76.8) |
*Data are presented by n (%). ER=Emergency room, ICU=Intensive care unit, OPD=Outpatient department, CXR=Chest X ray, MERS-CoV=Middle east respiratory syndrome-coronavirus
The overall in-hospital mortality rate was 13.8%. Patients infected with MERS-CoV were significantly more likely to die compared to others (59.7% vs. 17.7% in H1N1, 14.5% in Influenza A (non-H1N1), and 8.1% in influenza B; P < 0.001); [Figure 2].
Figure 2.
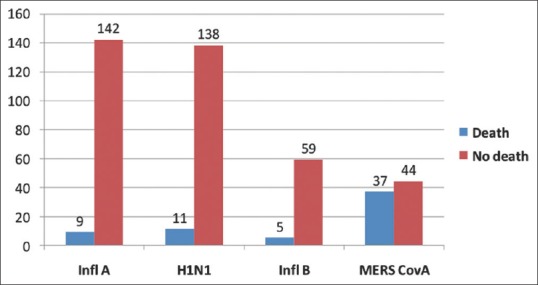
Bar chart presenting the frequency number of death among the studied confirmed viral pneumonia by type of associated virus January, 2012–October, 2015 (total: 448 cases)
The impact of baseline characteristics and type of the virus on mortality was assessed in univariate and binary variates analysis [Tables 3 and 4]. Age >65 years (odds ratio [OR]: 5.4; P = 0.0001), male sex (OR: 2.3; P = 0.003), heart failure (OR: 3.4, P < 0.001). The number of the comorbidities was proportionately related to mortality [Table 5].
Table 3.
Factors associated with the risk of death among the studied viral pneumonia confirmed cases (n=448)-univariate analysis
| Factor* | Died (n=62) | Alive (n=386) | OR | P |
|---|---|---|---|---|
| Age >65 years | 45 (72.5) | 127 (32.9) | 5.4 | <0.0001 |
| Sex (male) | 43 (69.4) | 189 (49.2) | 2.3 | 0.003 |
| Dialysis | 9 (14.5) | 27 (7.0) | 1.7 | 0.06 |
| CKD | 20 (32.3) | 44 (11.6) | 3.3 | <0.0001 |
| Chronic lung disease | 8 (12.9) | 77 (20.1) | 0.54 | 0.12 |
| Transplant | 2 (3.2) | 8 (2.1) | 1.5 | 0.63 |
| HF | 14 (22.6) | 29 (7.6) | 3.4 | <0.0001 |
| Cancer | 7 (11.3) | 11 (2.9) | 4.1 | 0.003 |
| IHD | 22 (35.5) | 76 (19.8) | 2.1 | 0.01 |
| Bed bound | 20 (32.3) | 26 (6.8) | 6.0 | <0.0001 |
| HTN | 49 (79.0) | 163 (42.4) | 4.6 | <0.0001 |
| DM | 41 (66.1) | 151 (39.3) | 2.7 | 0.001 |
| Stroke | 12 (19.4) | 20 (5.2) | 4.7 | <0.0001 |
| Liver disease | 3 (4.8) | 12 (3.1) | 1.5 | 0.55 |
| MERS-CoV | 37 (59.8) | 44 (11.4) | 11.4 | <0.000 |
*Data are presented by n (%). CKD=Chronic kidney disease, HF=Heart failure, IHD=Ischemic heart disease, HTN=Hypertension, DM=Diabetes mellitus, OR=Odds ratio, MERS-CoV=Middle east respiratory syndrome-coronavirus
Table 4.
Binary multivariate analysis for predictors of death in patients with viral pneumonia
| Variable | OR | P | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.015 | 0.601 | 0.959 | 1.075 |
| Gender | 2.367 | 0.349 | 0.070 | 2.564 |
| WBC | 1.027 | 0.374 | 0.968 | 1.090 |
| Hb | 0.659 | 0.032 | 0.451 | 0.964 |
| Platelet | 1.005 | 0.206 | 0.997 | 1.014 |
| CRP | 1.006 | 0.227 | 0.996 | 1.015 |
| CPK | 1.000 | 0.439 | 0.999 | 1.002 |
| CURB score | 1.779 | 0.051 | 0.998 | 3.170 |
| DM | 1.295 | 0.819 | 0.142 | 11.826 |
| HTN | 1.932 | 0.634 | 0.129 | 29.039 |
| CKD | 1.135 | 0.904 | 0.145 | 8.854 |
| HF | 2.623 | 0.261 | 0.488 | 14.104 |
| MERS versus non-MERS | 16.34 | 0.002 | 2.735 | 97.632 |
| Dialysis | 1.686 | 0.625 | 0.208 | 13.669 |
| Cancer | 3.577 | 0.299 | 0.322 | 39.731 |
| Bed ridden | 1.185 | 0.859 | 0.182 | 7.716 |
| Stroke | 2.344 | 0.413 | 0.304 | 18.057 |
DM=Diabetes mellitus, HTN=Hypertension, CKD=Chronic kidney disease, HF=Heart failure, MERS=Middle east respiratory syndrome, CI=Confidence interval, OR=Odds ratio, Hb=Hemoglobin, WBC=White blood cell, CRP=C-reactive protein, CPK=Creatine phosphokinase
Table 5.
Number of comorbidities and the risk of death among the studied viral pneumonia confirmed cases (n=448)
| Factor | Died (n=62) | Alive (n=386) | OR | P |
|---|---|---|---|---|
| One comorbidity | 4 | 40 | 1.84 | 0.10 |
| Two comorbidities | 15 | 76 | 4.12 | <0.0001 |
| Three comorbidities | 5 | 52 | 5.66 | <0.0001 |
| Four comorbidities | 8 | 30 | 5.52 | <0.0001 |
| More than four comorbidities | 11 | 10 | 20.2 | <0.0001 |
OR=Odds ratio
Discussion
Respiratory viruses, including influenza, have received more attention compared to other causes of pneumonia particularly after the emergence of the new H1N1 and MERS-CoV. In this cohort, pneumonia caused by MERS CoV-infection was more prevalent among older patients. A similar finding was also reported in studies from other Middle-East countries including UAE, Qatar, Oman, Jordan, Kuwait, and Yemen.[15] On the contrary, H1N1 pneumonia affects patients at a slightly younger age group.[16,17,18,19] It was noted during most pandemics that the age distribution of severe influenza-related pneumonia exhibits a U-shaped pattern, with young and the elderly patients were most frequently affected.[20,21] In this cohort, males were more likely to be infected. This is consistent with a similar finding reported by others,[15,16,17,18,19] although a recent report showed more MERS-CoV infection among female healthcare workers.[22]
The most common radiological finding in this study was bilateral lung infiltrate and that was mostly observed among MERS-CoV (94.1%) and H1N1 (86.7%) associated infections. The pattern of interstitial infiltrates with patchy distribution on chest radiograph was sometimes useful in differentiating viral from bacterial community-acquired pneumonia.[23] These changes, however, were not universal. Similarly, the presence of pleural effusion predicts bacterial infection,[24] and that was not reported in any of the studied cases.
The depicted epidemic curve in this study showed that the incidence of viral pneumonia associated with influenza virus and MERS-CoV infection was fluctuating all over the studied years. Studying the incidence of infection by season showed a steep rise in the curve during the period from October to December for influenza A (both non-H1N1 and H1N1), and in the period from January to March for MERS-CoV. This finding was reported by García-García et al.,[24] and Johnstone et al.[25] and it was included in H1N1 and MERS-CoV reports from WHO[7,11] and CDC.[6] The cause of this seasonal fluctuation is not clearly understood; however, one possible explanation is that the temperature of early spring and autumn in Arabian Peninsula is not high enough to destroy the virus,[26] knowing that MERS-CoV can be completely inactivated by heat treatment at 50°C for 30 min.[27]
Similar to mortality data and its predictors found in this study, the previously reported cohort of MERS-CoV patients found that age >65 years was an independent predictor of mortality while concomitant infections and low serum albumin were predictors for ICU admission.[28] In other recently published studies, older age, renal failure, diabetes, and hypertension were associated with increased mortality.[29,30] The associated comorbidities reported in our study, as well as other reports, were found to increase the risk of complications and death, among patients with H1N1 and MERS-CoV pneumonia.[31] Severe cases of influenza are more likely to occur with cardiovascular diseases (OR: 2.9), hypertension with (OR: 1.5), and neuromuscular disease with (OR: 2.7).[32] In older studies during the 1968–1969 pandemic, approximately three-quarters of the patients admitted to the hospital with pneumonia had underlying chronic medical conditions,[33] while in another large cohort, a history of heart or lung disease, diabetes mellitus, rheumatologic disease, renal disease, or dementia/stroke predicted an increased risk of pneumonia and influenza-related hospitalization.[34] The risk of mortality was found to progressively increase among cases with multiple comorbidities particularly among cases having more than one comorbidity. Unexpectedly, the presence of pre-existing lung disease was not found to affect the outcome in our study. Clearly, identification of risk factors associated with increased mortality is vital to identify candidates for potential future vaccines and therapy.
This study, despite its retrospective nature, is one of the largest studies describing viral pneumonia secondary to influenza and MERS-CoV infections in the Kingdom of Saudi Arabia. It covered various demographic, clinical, radiological, and laboratory characteristics of the patients and analyzed the predictors of poor outcome. However, the data emerged from a single center with a unique population might not reflect the whole spectrum of the disease.
Conclusion
Viral pneumonia remains a major health problem, particularly after emergence of new viruses. The fatality rate was high particularly among cases associated with MERS-COV infection. The most important predictors of death among these patients were old age, male sex, and associated comorbidities. Finally, routine testing for newly emergent viruses may be warranted for adults who have been hospitalized with pneumonia.
Study approval was granted from PSMMC IRB.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to express our thanks and appreciation to Ms. Wedad Al-Sufayani for helping in files collection and organizations.
References
- 1.File TM. Community-acquired pneumonia. Lancet. 2003;362:1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galván JM, Rajas O, Aspa J. Review of non-bacterial infections in respiratory medicine: Viral pneumonia. Arch Bronconeumol. 2015;51:590–7. doi: 10.1016/j.arbr.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangil A, Calbo E, Robles A, Benet S, Viladot ME, Pascual V, et al. Aetiology of community-acquired pneumonia among adults in an H1N1 pandemic year: The role of respiratory viruses. Eur J Clin Microbiol Infect Dis. 2012;31:2765–72. doi: 10.1007/s10096-012-1626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 5.Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–7. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. [Last accessed on 2017 Feb 21];CDC Health Update: Swine Influenza A(H1N1) Update: Influenza Activity- United States. Centers for Disease Control and Prevention; 29 September, 8 February, 2013. 2014 [Google Scholar]
- 7.World Health Organization. Distribution of Con- Firmed Cases of Influenza A(H1N1) by Age. WHO European Region. [Last accessed on 2009 Apr/May]. Available from: http://www.euro.who.int/influenza/AH1N0090508_5 .
- 8.Novel Swine-Origin Influenza A(H1N1) Virus Investigation Team. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A(H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 9.AlMazroa MA, Memish ZA, AlWadey AM. Pandemic influenza A(H1N1) in Saudi Arabia: Description of the first one hundred cases. Ann Saudi Med. 2010;30:11–4. doi: 10.4103/0256-4947.59366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. “Acute Respiratory Update”. [Last accessed on 2017 Feb 21]. Available from: http://www.who.int/vac-Cine_Research/diseases/ari/en/index5 .
- 11.World Health Organization. Middle East respiratory Syndrome Coronavirus (MERS-CoV) [Last accessed on 2017 Feb 21]. Available from: http://www.who.int/emergencies/mers-cov/en .
- 12.Alraddadi BM, Watson JT, Almarashi A, Abedi GR, Turkistani A, Sadran M, et al. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;13:752–61. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balkhy HH, Alenazi TH, Alshamrani MM, Baffoe-Bonnie H, Arabi Y, Hijazi R, et al. Description of a hospital outbreak of middle east respiratory syndrome in a large tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2016;37:1147–55. doi: 10.1017/ice.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bialek SR, Allen D, Alvarado-Ramy F, Arthur R, Balajee A, Bell D, et al. First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-coV) infection in the United States, updated information on the epidemiology of MERS-coV infection, and guidance for the public, clinicians, and public health authorities – May 2014. MMWR Morb Mortal Wkly Rep. 2014;63:431–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Delaney JW, Fowler RA. 2009 influenza A(H1N1): A clinical review. Hosp Pract (1995) 2010;38:74–81. [PubMed] [Google Scholar]
- 17.Dee S, Jayathissa S. Clinical and epidemiological characteristics of the hospitalised patients due to pandemic H1N1 2009 viral infection: Experience at Hutt hospital, New Zealand. N Z Med J. 2010;123:45–53. [PubMed] [Google Scholar]
- 18.Appuhamy RD, Beard FH, Phung HN, Selvey CE, Birrell FA, Culleton TH, et al. The changing phases of pandemic (H1N1) 2009 in Queensland: An overview of public health actions and epidemiology. Med J Aust. 2010;192:94–7. doi: 10.5694/j.1326-5377.2010.tb03427.x. [DOI] [PubMed] [Google Scholar]
- 19.Lan YC, Su MC, Chen CH, Huang SH, Chen WL, Tien N, et al. Epidemiology of pandemic influenza A/H1N1 virus during 2009-2010 in Taiwan. Virus Res. 2013;177:46–54. doi: 10.1016/j.virusres.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Glezen WP. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- 21.Serfling RE, Sherman IL, Houseworth WJ. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957-58, 1960 and 1963. Am J Epidemiol. 1967;86:433–41. doi: 10.1093/oxfordjournals.aje.a120753. [DOI] [PubMed] [Google Scholar]
- 22.Alsahafi AJ, Cheng AC. The epidemiology of Middle East respiratory syndrome coronavirus in the Kingdom of Saudi Arabia, 2012-2015. Int J Infect Dis. 2016;45:1–4. doi: 10.1016/j.ijid.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377:1264–75. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-García ML, Calvo C, Pozo F, Villadangos PA, Pérez-Breña P, Casas I, et al. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31:808–13. doi: 10.1097/INF.0b013e3182568c67. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: Prevalence, pathogens, and presentation. Chest. 2008;134:1141–8. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sizun J, Yu MW, Talbot PJ. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: A possible source ofhospital-acquired infections. J Hosp Infect. 2000;46:55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW, et al. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: A single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–6. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N, et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: A retrospective study. J Antimicrob Chemother. 2015;70:2129–32. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alraddadi B, Bawareth N, Omar H, Alsalmi H, Alshukairi A, Qushmaq I, et al. Patient characteristics infected with Middle East respiratory syndrome coronavirus infection in a tertiary hospital. Ann Thorac Med. 2016;11:128–31. doi: 10.4103/1817-1737.180027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badawi A, Ryoo SG. Prevalence of diabetes in the 2009 influenza A(H1N1) and the Middle East respiratory syndrome coronavirus: A systematic review and meta-analysis. J Public Health Res. 2016;5:733. doi: 10.4081/jphr.2016.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP, et al. Populations at risk for severe or complicated influenza illness: Systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bisno AL, Griffin JP, Van Epps KA, Niell HB, Rytel MW. Pneumonia and Hong Kong influenza: A prospective study of the 1968-1969 epidemic. Am J Med Sci. 1971;261:251–63. doi: 10.1097/00000441-197105000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Nichol KL, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch Intern Med. 1998;158:1769–76. doi: 10.1001/archinte.158.16.1769. [DOI] [PubMed] [Google Scholar]


