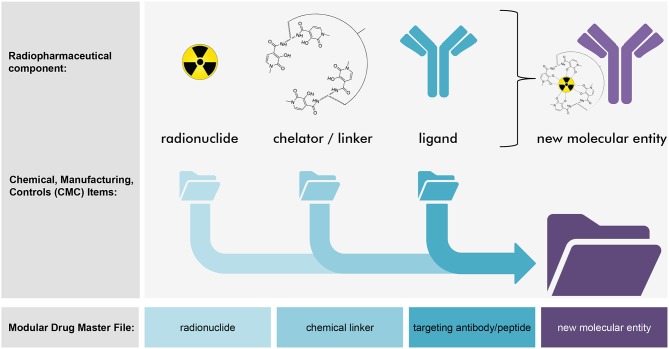
Figure 2.
Strategy for modular radiopharmaceutical drug master files. Depicted are NCI's thoughts on a conceptual strategy for modular radiopharmaceutical drug master files (DMFs). This illustration describes a radiopharmaceutical from the conjugate class. Here, the new molecular entity is made up of three components—a radionuclide, a chemical chelator or linker, and a targeting ligand (such as the depicted antibody). Each component might have its own individual DMF that details its individual chemistry, manufacturing, and controls. By cross-reference, a modular radiopharmaceutical DMF might detail the new molecular entity's chemistry, manufacturing, and controls without duplicating forms, filings, and effort. In concept, this approach speeds clinical development of a radiopharmaceutical that might be considered a new molecular entity by regulatory agencies.