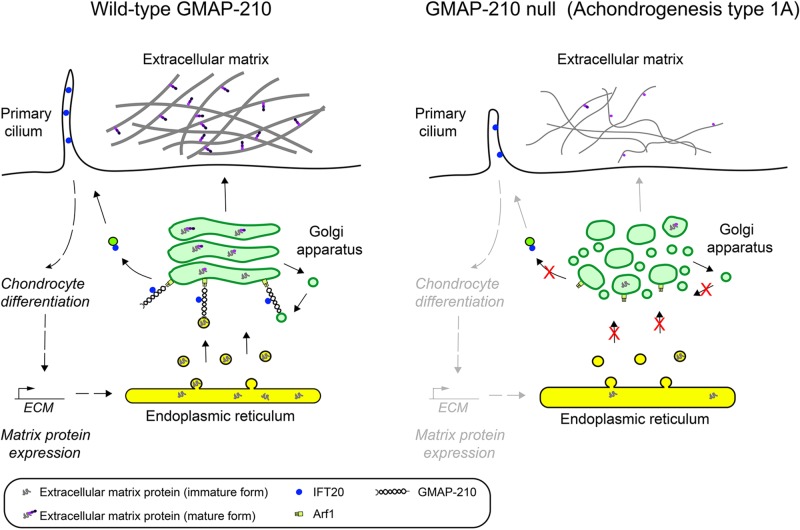
FIGURE 4.
Loss of GMAP-210 manifests as skeletal dysplasia in humans with achondrogenesis type 1A. GMAP-210 tethers ER derived vesicles containing newly synthesized extracellular matrix proteins en route to the Golgi apparatus. It also tethers intra-Golgi vesicles responsible for recycling Golgi enzymes that are important for modifying secretory cargo, including extracellular matrix proteins, which is required for their maturation. Following transit through the Golgi the fully modified matrix proteins are secreted and assembled into the extracellular matrix. In addition, GMAP-210 anchors IFT20 to the Golgi, which is required for the IFT20-dependent transport of cargo proteins to the cilium. This is necessary for ciliary signaling, which helps maintain the differentiation status of chondrocytes and their ability to synthesize and secrete large amount of matrix proteins. Upon loss of GMAP-210, as occurs in achondrogenesis type 1A (ACG1A), the loss of vesicle tethering at the cis-Golgi results in a deficit in both transport and modification of extracellular matrix proteins, which in turn causes a defective cell matrix to form outside the cells. This manifests as the severe skeletal dysplasia achondrogenesis Type 1A. There is also a loss of IFT20-dependent transport of cargo proteins to the cilium, resulting in defective ciliary function and impaired cell differentiation, such that matrix production is also decreased, further compounding the phenotype.