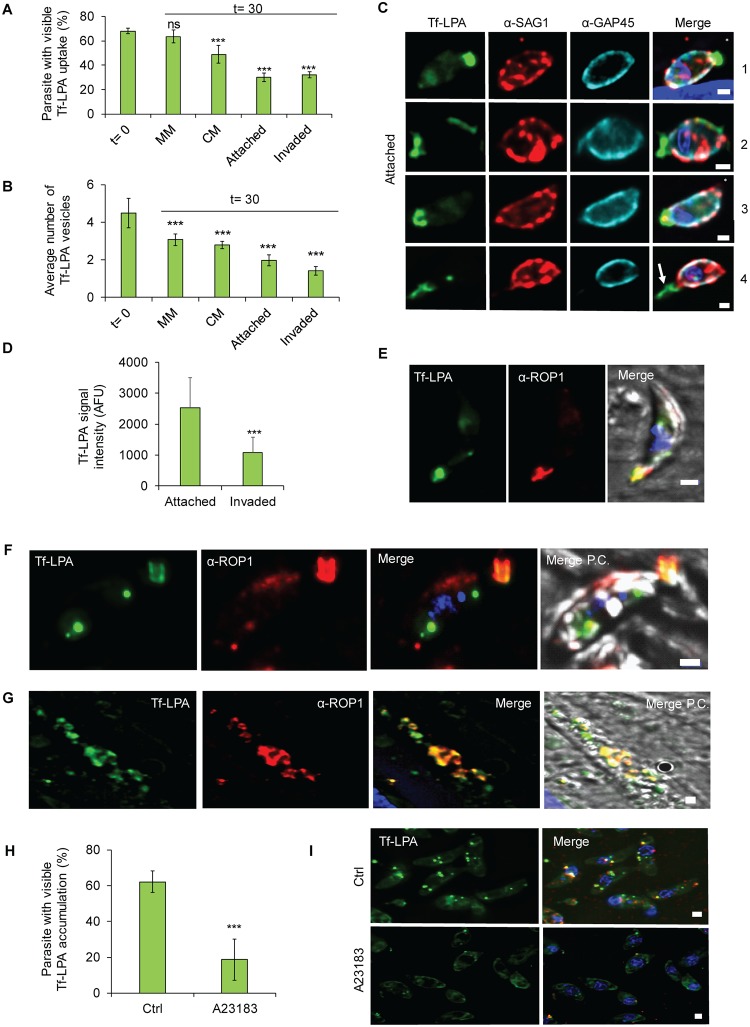
Fig 4. Tf-LPA follow an endocytic-exocytic pathway.
(A-E) Exocytosis of Tf-LPA was evaluated 30 minutes after placing parasites under three conditions: MM, CM, and CM with host cells. In the latter case, invaded and attached parasites were analysed separately. A clear diminishing of the signal is observed over time in stimulating conditions, demonstrating secretion of previously endocytosed Tf-LPA. (A) Percentage of Tf-LPA–positive parasites and (B) average number of vesicles were calculated. Mean values of three independent assays are shown ± SD. ***p < 0.001 in a two-tailed Student t test. (C) Illustration of parasites transferred onto host cells. SAG1 staining (prior to permeabilisation) was used to differentiate intra- from extracellular parasites. Scale bar, 1 μm. From top to bottom: attached parasites: (1) parasite with apical accumulation of Tf-LPA, (2) parasite with partial membrane labelling with Tf-LPA, (3) parasite with Tf-LPA accumulated at the basal pole, (4) parasite with Tf-LPA left in the trail. Asterisk (*) indicates the apical pole of the parasite. (D) Quantification of Tf-LPA signal intensity between attached and invaded parasites. (E) IFA using α-ROP1 confirming the apical presence of Tf-LPA. (F, G) Secretion of Tf-LPA inside evacuoles was tested. Colocalisation between Tf-LPA (green) and α-ROP1 (red), as observed in evacuoles. (H-I) Impact of secretion stimulation on the accumulation of Tf-LPA vesicles. Exocytosis was stimulated using the calcium ionophore A23187. (H) The uptake Tf-LPA under the presence or absence of A23187 was quantified. Mean values of three independent assays are shown ± SD. ***p < 0.001 in a two-tailed Student t test. (I) Representative pictures of both uptake conditions. Scale bar, 1 μm. For each bar graph, the corresponding data can be found in S1 Data. AFU, arbitrary fluorescent unit; CM, complete media; Ctrl, control; IFA, immunofluorescence assay; MM, minimal media; P.C., phase contrast; Tf-LPA, Top-Fluor lysophosphatidic acid.