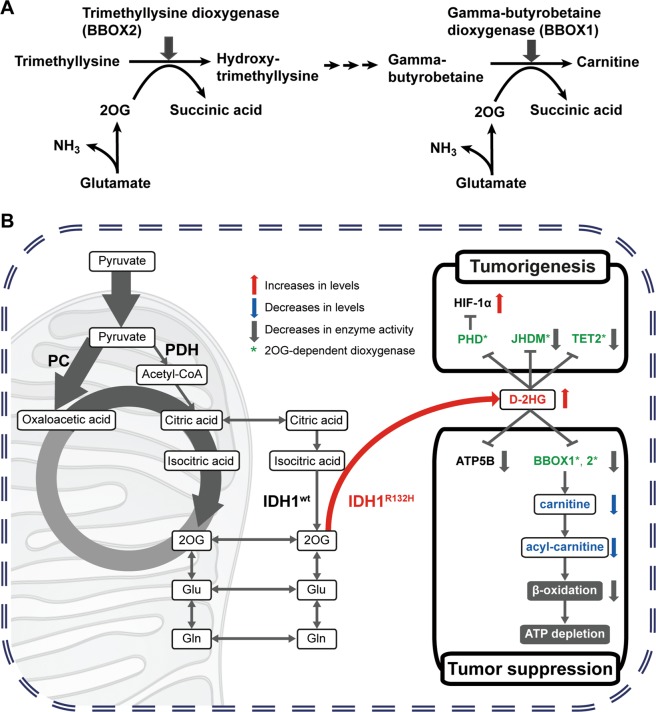
Figure 5.
The hypothesis of the mechanism of IDH mutant gliomas. (A) 2OG-dependent dioxygenases in carnitine biosynthesis. There are two 2OG-dependent dioxygenases in the carnitine synthetic pathway. Carnitine is produced from gamma-butyrobetaine by gamma-butyrobetaine dioxygenase (BBOX 1) that is the last enzyme in the carnitine synthetic pathway. Trimethyllysine is converted into hydroxytrimethyllysine by trimethyllysine dioxygenase (BBOX 2) that is the first enzyme in the carnitine synthetic pathway. In these chemical reactions, 2OG acts as a cofactor. Reduced levels of 2OG in IDH mutant clinical tissues may lead to decreased carnitine synthesis through inhibition of these enzyme activities. (B) The proposed hypothesis for the mechanism of metabolism in IDH mutant tissues. The IDH mutation has two effects: a tumorigenic effect and a tumor suppressive effect. IDH1 mutant gliomas produce D-2-hydroxyglutarate (D-2HG), resulting in inhibition of 2OG synthesis and downstream metabolic intermediates in the TCA cycle. As a tumorigenic effect, D-2HG produced by mutant IDH causes activation of HIF-1α through the inhibition of PHD (prolyl hydroxylases) activity and the direct inhibition of JHDM (Jumonji C-domain-containing histone demethylases) and TET2 activities. As a tumor suppressive effect, D-2HG inhibits ATP production through at least two ways. D-2HG directly inhibits ATP synthase activity by interaction with ATP5B (ATP synthase β subunit) and reduces ATP production. In addition, β-oxidation in clinical gliomas with IDH mutation is suppressed due to the reduction of carnitine levels. BBOX 1 and BBOX 2 in the carnitine synthetic pathway may be inhibited by high levels of D-2HG and lower levels of 2OG. Oxaloacetic acid could be supplied from pyruvate by pyruvate carboxylase (PC) as an anaplerotic reaction.