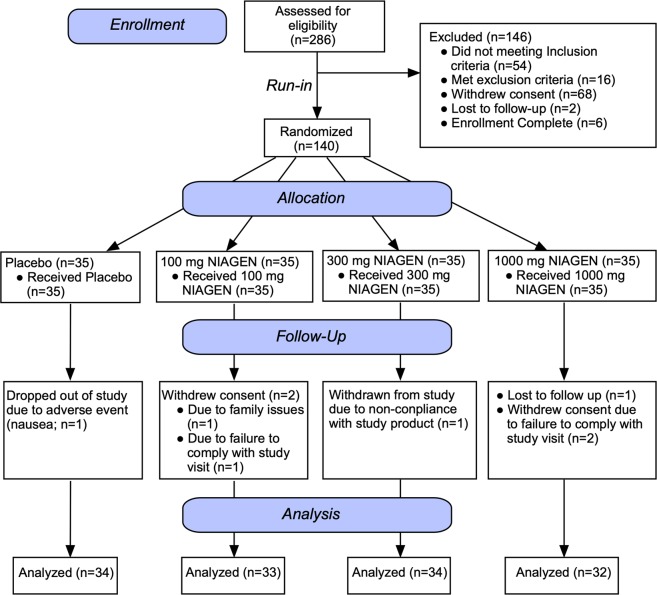
Figure 2.
Disposition of the study participants. Two hundred and eighty-six men and women were screened for eligibility. One hundred and forty subjects met the eligibility criteria and were enrolled in the study. After the 2-week run-in (Day 0), the subjects were randomized to one of four treatment groups (Placebo, 100 mg, 300 mg, or 1000 mg NIAGEN per day; n = 35/group). Over the course of the 56-day supplementation period, one subject withdrew from the placebo-treated group due to an adverse event, two subjects withdrew consent in the 100 mg NIAGEN treated group, one subject was withdrawn from the 300 mg NIAGEN-treated group and two subjects withdrew consent and one was lost to follow-up in the 1000 mg NIAGEN-treated group.