Abstract
Background
Extracellular Glucose-regulated protein94 (Grp94) is linked to pathological conditions disrupting the obligatory intracellular location of this Heat Shock Protein (HSP). In plasma, Grp94 is linked to IgG in complexes that drive adverse effects on vascular cells and are biomarker of gastro-intestinal cancer. By blocking ATP site in different HSPs, purine-scaffold inhibitors are used as promising anti-cancer compounds, but their effects on vasculature are not known.
Methods
We tested the capacity of two purine-scaffold inhibitors, PU-H71 and PU-WS13, to prevent the binding of Grp94 to IgG and to antagonize the effects of Grp94 and native Grp94-IgG complexes on HUVECs in different experimental conditions.
Results
PU-H71 and PU-WS13 blocked Grp94 and the formation of Grp94-IgG complexes in absence of cells. Instead, in presence of HUVECs rather than Grp94 PU-inhibitors targeted cells causing stimulation of Akt and VEGF pathways and displaying angiogenic-like effects similar to, although less intense than that provoked by Grp94 and Grp94-IgG complexes. Unlike Grp94 and Grp94-IgG complexes, PU-inhibitors also activated the purinergic pathway and increased the expression of the ATP receptor P2X7. Effects of PU-inhibitors on HUVECs were reversed by ATP and in presence of ATP PU-inhibitors were again able to block Grp94.
Conclusions
PU-inhibitors can display direct effects on endothelial cells by targeting the ATP receptor P2X7. In absence of ATP, PU-inhibitors preferentially bind to cells rather than Grp94. ATP antagonizes the PU-inhibitor binding to cells thus restoring the capacity to block Grp94 and Grp94-IgG complex formation. Results have implications for enhancing the therapeutic efficacy of PU-inhibitors against circulating pathogenic Grp94.
Keywords: Endothelial cells, Purine-based inhibitors, Heat shock proteins, Angiogenic proteins, Signal transduction pathways
Highlights
-
•
Extracellular Grp94 forms pathogenic complexes with IgG.
-
•
PU-inhibitors block the Grp94-IgG complex formation in absence of cells.
-
•
PU-inhibitors target cells and activate the purinergic pathway.
-
•
Effects of PU-inhibitors on cells are reversed by ATP.
-
•
ATP restores the capacity of PU-inhibitors to block the Grp94-IgG complex formation.
1. Introduction
In the strenuous search for effective and safe anticancer therapy, a particular effort has being paid in recent years to identify drugs that target members of the heat shock protein (HSP)90 family that play a pivotal role in the process of malignant transformation of cells [1]. The expression of chaperones of this family, in particular HSP90 and glucose-regulated protein94 (Grp94), the most abundant endoplasmic-resident HSP, is significantly increased in different types of both solid [[2], [3], [4]] and hematological tumors [5,6]. While up-regulation of HSP90 is the response to the increased demand to chaperone pro-mitotic and angiogenic client proteins actively synthesized by cancer cells [7], the higher expression and membrane-bound location of Grp94 have only recently been related to chaperoning tumor-specific client proteins [8].
The intense studies on the structure and biochemical properties of HSP90 and Grp94 have permitted to discover and synthesize both pan-HSP90 [9] and paralog-specific inhibitors that target the ATP binding site in these HSPs [8,10]. The basic concept to the research is that occupancy of the ATP binding site by purine-scaffold derivative compounds in place of the natural ligand, can lead to structural conformations of the HSPs that block the chaperone activity, causing downstream defective functioning of the tumor-related client proteins. Among the pan-HSP90 inhibitors, PU-H71 turned out to be the most promising anti-tumor compound for the cure of different types of cancer [[11], [12], [13]], whereas PU-WS13 has emerged as Grp94-specific inhibitor that besides targeting this HSP would have the advantage to overcome the feed-back up-regulation of anti-apoptotic HSP70, hallmark of HSP90 inhibition driven by pan-inhibitor PU-H71 [8,14,15].
Although many different HSP90 inhibitors have been developed so far, and some of them also entered into clinical evaluation, the progress made for using this class of compounds as effective anti-cancer drugs in clinical practice has been limited by failures [16]. One possible reason might depend on being these molecules almost exclusively tested in cancer cells and the mechanism(s) by which they work in both tumor and normal tissue environment has not investigated in detail. It has also become clear that both HSP90 and Grp94 cannot any longer be considered as individual anti-tumor pharmacological target, since they operate in supra-molecular complexes that have functional relevance for the survival of tumor cells and show responsiveness to purine-scaffold HSP inhibitors [17]. While the recent acquisitions about the role of chaperones operating in concert rather than singularly in supporting tumor growth are relevant to shed light on the metabolism of tumor cell in order to identify tumors susceptible to specific PU-inhibitors, the question remains as to whether and how PU-inhibitors could target chaperones once these are expressed on tumor cells or liberated from the tumor into circulation. The question especially pertains to Grp94 that is considered one of the most diffuse tumor antigen and a more reliable indicator of tumor malignancy [18,19]. When present in the extra-cellular milieu, Grp94 is found exclusively bound to IgG, as it has first been observed in type 1 diabetes in which Grp94 is liberated in the plasma by autoimmune process [20], and the complexes that it forms with IgG are particularly stable and can be detected long after the onset of disease [21]. It has been demonstrated that both native Grp94-IgG complexes isolated from plasma of diabetic patients and surrogate complexes formed in vitro by incubating Grp94 with pre-immune IgG show qualitatively similar angiogenic-like effects on human umbilical vein endothelial cells (HUVECs) by activating differentiation-specific cellular pathways [22,23]. The finding that Grp94 in complexes with IgG has the capacity to sustain and propagate the morphological alteration on vascular cells by autocrine/paracrine mechanism [23], together with the observation that Grp94-IgG complexes circulate in plasma of cancer plasma as a marker of the disease [24] support the possibility that alterations induced by these complexes on the vasculature can contribute to the growth and dissemination of the tumor. It would be therefore of particular interest to see whether purine-scaffold compounds can either antagonize Grp94 when in complex with IgG, or prevent Grp94 binding to IgG, thus representing an useful pharmacological option for antagonizing the unwanted effects driven on the vasculature by Grp94-IgG complexes. In addition, considering the particular chemical structure of these compounds, the question also arises as to whether they might exert the antagonistic effect by a mechanism other than the predicted inhibition of the ATP binding site in Grp94.
To address some of these questions, in this work we tested two PU-inhibitors, the pan-HSP90 inhibitor PU-H71 and the Grp94-selective PU-WS13 on HUVECs in different experimental conditions, and investigated the mechanism by which these inhibitors might counteract the effects driven on endothelial cells by Grp94 used both alone and in stable complexes with IgG as those isolated from plasma of diabetic subjects. Results indicate that, besides targeting the ATP-binding site in Grp94, PU-inhibitors exert direct effects on HUVECs that involve P2X7 receptor (P2X7R) and the purinergic pathway. In the context of a cellular setting, antagonism of Grp94 and prevention of the Grp94-IgG complex formation by PU-inhibitors crucially depend on both ATP and PU-inhibitor concentration.
2. Materials and methods
2.1. Reagents and antibodies
Collagenase (Worthington Biochemical Corporation, Lakewood, NJ), Endothelial Basal Medium (EBM), Fetal Bovine Serum (FBS low IgG, <100 μg/ml), Penicillin, Streptomycin, recombinant human epidermal growth factor, bovine brain extract and hydrocortisone were from Lonza (Walkersville, MD, USA). Protease inhibitor cocktail tablets were from Roche Diagnostics (Roche Diagnostics GmbH, Mannheim, Germany) and phosphatase inhibitors from Serva (Serva Electrophoresis GmbH, Heidelberg, Germany). The following primary anti-human antibodies (Abs) were used: anti phospho and total-p38 rabbit monoclonal Abs, anti phospho ERK1/2 rabbit polyclonal Abs, anti phospho Akt rabbit monoclonal Abs from Cell Signaling Technologies (Cell Signaling Technology, Inc., Danver, MA, USA), anti-ERK1/2 and anti-Akt1/2/3 rabbit polyclonal Abs, anti-P2X7R goat polyclonal, anti-VEGF and anti-HSP90α/β rabbit polyclonal, anti-HSP70 mouse monoclonal from Santa Cruz (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti β actin mouse monoclonal from Sigma-Aldrich (Sigma-Aldrich, St. Luis, MO, USA). Secondary Abs were HRP-conjugated (KPL, Gaithersburg, MD, USA), and biotin-conjugated (Bethyl Laboratories Inc.) coupled with HRP-conjugated streptavidin (KPL). All other reagents were of analytical grade from Sigma-Aldrich and Fluka (Fluka Chemie, GmbH, Buchs, Switzerland). PU-H71 and PU-WS13 were obtained as reported [8].
2.2. Experiments with human plasma
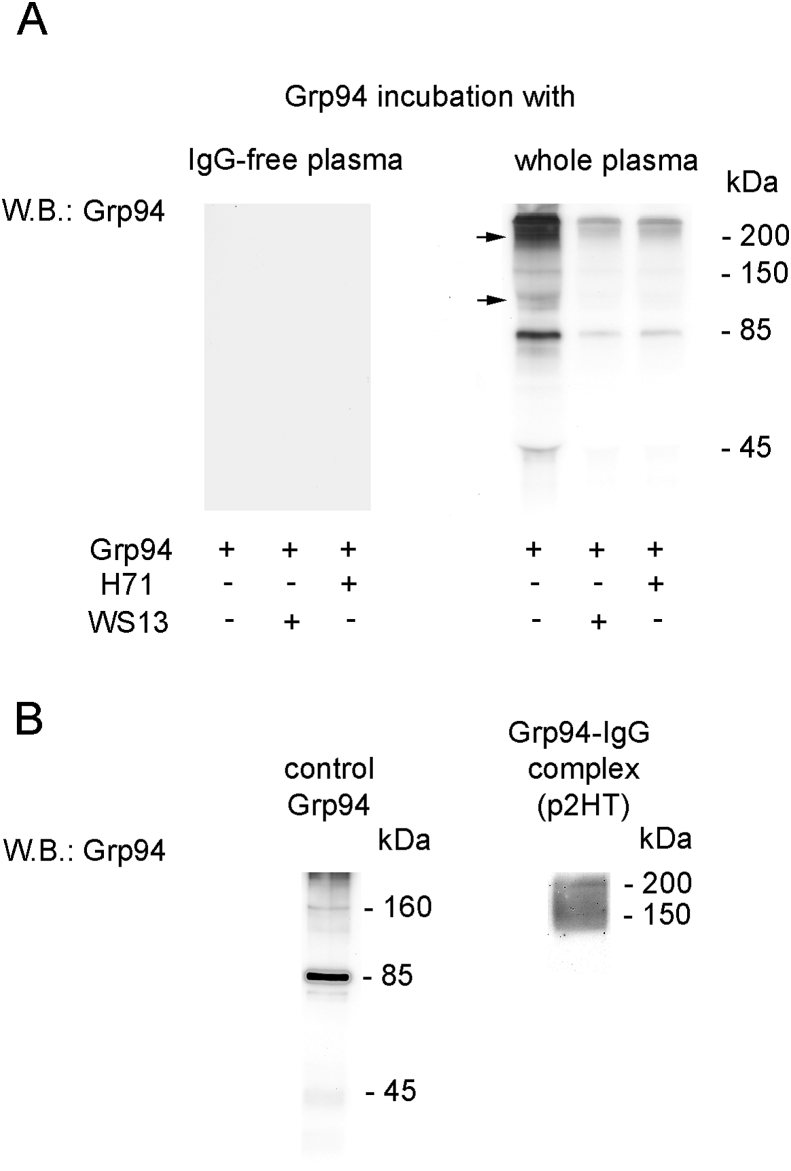
The study protocol for obtaining blood from both Type1 diabetic and control healthy subjects was approved by the Ethics Committee (Comitato Etico per la Sperimentazione, Regione Veneto), and both patients and healthy subjects gave their written informed consent for drawing 10-ml blood. Four Type1 diabetic subjects with mean age (±SD) of 27.5 (±8.4) yr and a mean diabetes duration of 10.4 (±6.9) yr, and four age-matched, non-smokers volunteers with a mean age of 35.2 (±6.7) yr were recruited for having blood samples. For obtaining Grp94-IgG complexes from plasma of diabetic subjects, blood was centrifuged and protein concentration measured spectrophotometrically at 280 nm. Each plasma sample contributed an equal protein content to form a plasma pool (62 mg/ml in 20 mM phosphate buffer, pH 7.0) that was loaded on to a 1-ml HiTrap ProteinG HP column (Amersham Biosciences) applied to a fast liquid chromatography system (Pharmacia LKB, Uppsala, Sweden). In the flow-through peak plasma proteins other than IgG eluted with buffer A (20 mM phosphate, pH 7.0), whereas integer IgG were recovered with buffer B (0.1 M glycine, pH 2.5) in the second peak. To avoid protein denaturation due to acidity of buffer B, 200 μl of 1.0 M Tris-HCl, pH 9.0, were added to IgG fractions immediately after recovery. Peaks were ultra-filtered on Amicon Centriplus YM-3 and final concentration of IgG measured at 280 nm using the extinction coefficient of E280 = 0.145. A 20-μl sample of IgG (5–7 μg) were loaded on to a 8.5% polyacrylamide gel in non reducing conditions for being processed in SDS-PAGE followed by Western blotting for Grp94 and IgG.
Plasma from the blood of healthy subjects was used to obtain Grp94-IgG complexes in vitro. Each sample contributed an equal content of plasma protein to form a pool that was loaded to a HiTrap ProteinG HP column as above for diabetic subjects. IgG-deprived plasma was obtained in the flow-through peak eluted from the HiTrap column, since it contained all plasma proteins (mostly HSA) other than IgG. Recombinant rabbit Grp94 was prepared as specified previously [25] and incubated both alone (160 μg/ml) and together with PU-inhibitors (1 μM PU-H71 and PU-WS13) in both whole human plasma (3.8 mg/ml proteins) and in IgG-deprived plasma (containing the same HSA concentration of whole plasma), in the final volume of 250 μl at 37 °C for 4.0 h. After incubation, a 10-μl aliquot of each sample was loaded in non-reducing conditions to an 8.5% polyacrylamide gel for being processed in SDS-PAGE followed by Western blotting for Grp94.
2.3. Cell cultures
HUVECs were cultured as specified [22] using at least four different cords for any cell preparation. Cells used at the passage 3–5 were maintained in endothelial basal medium (EBM) supplemented with 10% (v/v) FBS, 100 units/ml penicillin, 10 μg/ml streptomycin, 0.1% (v/v) rhEGF, 0.1% (v/v) hydrocortisone, 0.4% bovine brain extract and 0.1% (v/v) ascorbic acid, at 37 °C in a humidified 95% air, 5% CO2 atmosphere until cells reached sub-confluence. Cell culture purity was assessed by microscopic examination of the typical cobblestone morphology and by PE mouse anti-human CD31 monoclonal Abs (BD Pharmingen™, San Josè, CA, USA).
2.4. Cell proliferation assay
Proliferation was evaluated by means of the MTT test. HUVECs were seeded at the concentration of 7.5 × 104/ml in 96-well plates (200-μl wells) in EBM supplemented with 10% FBS. After over-night starvation in serum-free medium, cells were supplemented with a fresh aliquot (200 μl) of medium without and with either 1% human plasma or 1% FBS (in absence and presence of 0.5 mM ATP), and incubated without and with either Grp94 (100 ng/ml) or IgG (100 ng/ml) purified from the pool of diabetic plasmas (peak 2 from HT column containing native Grp94-IgG complexes). PU-H71 and PU-WS13, diluted in sterile water with 1.25% DMSO, were added at scalar concentrations (50 nM–1 μM for PU-H71, 250 nM to 1 μM for PU-WS13) together with Grp94 and p2HT to cells in triplicate wells. Vehicle alone was added in control wells. After incubation of both 6.0 h and 20 h, 20 μl of MTT (5 mg/ml in PBS) were added to each well and left in incubation for 4.0 h. The supernatant was then discarded and 100 μl of isopropanol contained 0.04 M HCl added for solubilization of formazan crystals. Absorbance was read at 570 nm as the value expressing the proliferation value.
2.5. Analysis of cell lysates
HUVECs (25 × 104/well) were seeded in 12-well plates (2 ml/well) in EBM supplemented with 10% FBS, and allowed to attach to well bottom for 24 h in a humidified 95% air and 5% CO2 atmosphere at 37 °C. Cells were then starved in fresh, serum-free medium for 5.0 h. After this time, a fresh aliquot (2 ml) of EBM, in both absence and presence of either 1% human plasma or 1% FBS (with and without 0.5 mM ATP), was added to cells with and without Grp94 (100 ng/ml) or p2HT (100 ng/ml, as above). After 1.0 h of incubation, PU-WS13 and PU-H71, diluted in sterile water with 1.25% DMSO, were added to the medium at the final concentration of 250 nM. Control cells were treated with the vehicle alone. Duplicate wells were used for each condition. At the end of incubation at both 6 and 20 h, medium was collected for separate analyses and cells washed with PBS, centrifuged for 10 min at 1500 rpm and lysed in 40 μl of RIPA buffer (Tris-HCl 50 mM pH 7.4, NaCl 150 mM, EDTA 1 mM, Triton X-100 1%, SDS 0.1%, sodium deoxycholate 1%) with the addition of protease and phosphatase inhibitors cocktail. Proteins were measured by the micro-BCA assay. Both pre-cast 4–20% poly-acrylamide gradient gels (Life Technologies, Carlsbad, CA, USA) and 8.5% polyacrylamide gels were used for the electrophoretic analysis of proteins (details are in the legends to the related figures). Proteins were then transferred on to a PVDF membrane for Western blot analysis for the above proteins and immunodetection performed with enhanced luminal-based chemiluminescent system (Immunological Sciences, Rome, Italy).
2.6. Analysis of media
Media from duplicate wells were collected, centrifuged for 10 min at 800×g to remove cell debris and extensively dialyzed against a volume excess of Milli-Q water. The lyophilized material was re-suspended in 40 μl of sample buffer (Tris-HCl 0.125 M, pH 6.8, glycerol 20% and SDS 2%) and analyzed in SDS-PAGE followed by Western blotting for immunodetection of the specified proteins.
2.7. Statistical analysis
Data of cell growth were presented as mean ± S.D. GraphPad Prism (GraphPad software, Inc. San Diego, CA, USA) was used for statistical analysis of data. Comparisons between group means were made by two-way analysis of variance (ANOVA). A P-value <0.05 was considered statistically significant.
3. Results
3.1. PU-inhibitors antagonize the Grp94-IgG complex formation in a-cellular setting
To see whether PU-H71 and PU-WS13 were able to inhibit the binding of Grp94 to IgG, a condition that might be exploited to prevent the complex formation in vivo, experiments were first set up in a-cellular setting in which PU- inhibitors were incubated together with Grp94 in human plasma, either whole or deprived of IgG (Fig. 1). Consistent with previously reported observations, Grp94 underwent a time- and temperature-dependent auto-proteolytic degradation [25] and after 4.0 h incubation at 37 °C in absence of IgG (but with all other plasma proteins present in the medium) no band of Grp94 was detected in the blotting (Fig. 1A; empty lanes). After incubation with whole plasma (Fig. 1A, lanes on right), Grp94 was instead detected at molecular masses higher than its molecular weight, consistent with the formation of big, SDS-resistant complexes with IgG, as also proved by co-positivity for IgG of the Grp94 bands (data not shown) [25]. PU-inhibitors added to the medium of whole plasma significantly reduced the intensity of immune reaction for Grp94 (Fig. 1A, lanes on right), thus proving that part of Grp94 was degraded as in IgG-deprived plasma. Results are consistent with having the binding of PU-H71 and PU-WS13 to the ATP-binding site caused a conformational shift in Grp94 sufficient to prevent any further binding to IgG. In this respect, the effect of PU-inhibitors is significantly different from that displayed by ATP that instead was able to stabilize the complex formation [25].
Fig. 1.
PU-inhibitors prevent the formation of complexes that Grp94 forms with IgG in human plasma. A. Recombinant rabbit Grp94 (160 μg/ml) was incubated (in a volume of 250 μl) for 4 h at 37 °C both alone and with PU-inhibitors (1 μM) in IgG-deprived (left) and whole human plasma (right), as specified in Methods. In the incubation medium with whole plasma the IgG concentration was about 4 μM, corresponding to a 2:1 M ratio with Grp94. An aliquot of 10 μl of each incubated sample was loaded in non-reducing conditions to an 8.5% polyacrylamide gel in SDS-PAGE followed by Western blotting for Grp94. B. Fresh, non incubated sample of Grp94 (1.6 μg) was processed in SDS-PAGE as above and tested for anti-Grp94 antibodies in Western blotting (left). Band at about 160 kDa corresponds to the dimer of Grp94 disappearing in reducing conditions (not shown). Right: Western blotting for Grp94 on the fraction of IgG purified from pooled diabetic plasma eluted in the peak 2 from the Protein G Hi-trap column, as detailed in Methods. Seven μg proteins were loaded in non-reducing conditions of sample. The broad band of between 150 and > 200 kDa also shows positivity for human IgG (not shown). Molecular masses are indicated on right of lanes.
3.2. PU-H71 and PU-WS13 differently affect viability and morphology of HUVECs in both absence and presence of Grp94 and native Grp94-IgG complexes
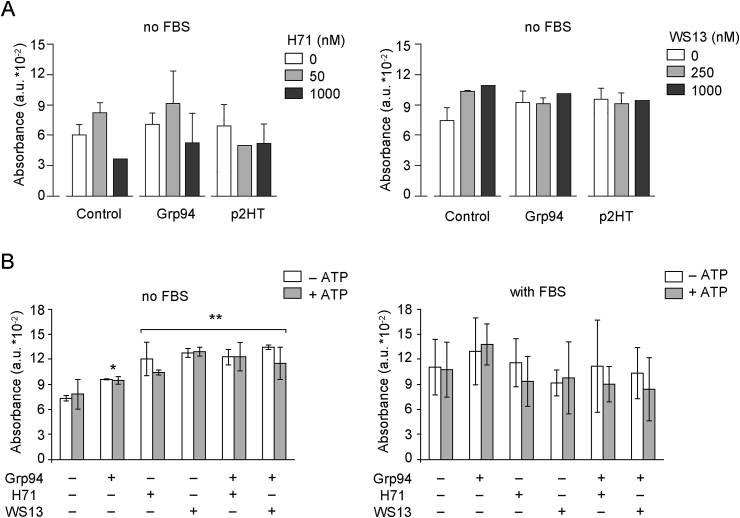
We then sought to investigate whether PU-inhibitors were able to antagonize the formation of Grp94-IgG complexes also in cell culture, thus preventing the well-known effects that both Grp94 alone and Grp94-IgG complexes exert on vascular cells [22,23]. To this aim, Grp94 was incubated both alone and with PU-inhibitors with HUVECs in absence and presence of human plasma (or FBS), and effects measured on cell viability and morphology. To also see whether PU-inhibitors could antagonize or reduce the effects displayed on vascular cells by already formed Grp94-IgG complexes, in other experiments HUVECs were incubated, in absence of plasma/serum, with native Grp94-IgG complexes obtained in the last step of IgG purification from pooled plasma of diabetic subjects (peak 2 from HiTrap column, henceforth p2HT). As predicted from previous results [23], Grp94 in diabetic plasma was found inextricably linked to IgG to form high-molecular mass and SDS-resistant complexes (Fig. 1B), similar to those formed experimentally (Fig. 1A). After 6 h incubation in absence of serum, both Grp94 alone and p2HT (100 ng/ml both) increased cell viability to a similar extent (29 ± 19.5% and 32 ± 18% for Grp94 and p2HT, respectively) (Fig. 2A). PU-inhibitors (50, 250 and 1000 nM) not only failed to antagonize the stimulatory effect of both Grp94 and p2HT, but on their own (PU-H71 at the lowest concentration, PU-WS13 at any concentration) increased cell viability markedly although this effect did not reach statistical significance (Fig. 2A). A likely explanation of these results was that PU-inhibitors, whose concentration in the medium by far exceeded that of Grp94 and p2HT, could also directly act on ATP receptors present on cell membrane.
Fig. 2.
Effects of PU-inhibitors on cell viability are independent of those of both Grp94 and native Grp94-IgG complexes. Cells were cultured and seeded as specified in Methods and after overnight starvation in serum-free medium cells were supplemented with fresh medium with and without 1% FBS and incubated with both Grp94 and p2HT (100 ng/ml each) in presence of PU-inhibitors, and after incubation of 6.0 h, MTT was added to evaluate the entity of viability expressed as absorbance (in arbitrary units) at 570 nm. A. Cells were cultured in triplicate wells and PU-inhibitors added at the indicated concentrations. Histograms represent the means (±SD) of measurements made in two separate experiments. B. Cells were cultured in quadruplicate wells in both absence (control) and presence of Grp94 and PU-inhibitors (250 nM each) with and without ATP (0.5 mM). Histograms are the means (±SD) of measurements made in three separate experiments. Asterisks mark the significant (*P < 0.05) and highly significant (**P < 0.001) difference with respect to the control (Two-way ANOVA).
Since purine-scaffold molecules compete with ATP at the ATP binding site in Grp94, and an increase in the extra-cellular ATP is reported to occur in different pathological conditions [26], in separate experiments we also used ATP (0.5 mM) to test whether ATP could antagonize the effects driven by Grp94 on HUVECs. It was thus confirmed that the significant stimulation of cell viability by Grp94 (30 ± 3.2%, p < 0.05) was not antagonized by PU-inhibitors (both at 250 nM) that on their own caused an even higher stimulation (64 ± 14% and 74 ± 1.3% for PU-H71 and PU-WS13, respectively, p < 0.001 compared to the control) (Fig. 2B, graph on left). The finding that, when added to Grp94, PU-inhibitors induced the same stimulation as that observed with PU-inhibitors alone, suggested that the stimulatory effect of Grp94 and PU-inhibitors was likely mediated by a different mechanism. ATP did not modify substantially this pattern so that only Grp94 and PU-inhibitors appeared to be responsible for the stimulation, without any interfering effect of ATP (two-way ANOVA, F = 17, p < 0.0001). In presence of FBS, a higher variability in the response was apparent so that no clear effect was any longer visible with both PU-inhibitors and Grp94, in absence and presence of ATP (Fig. 2B, graph on right).
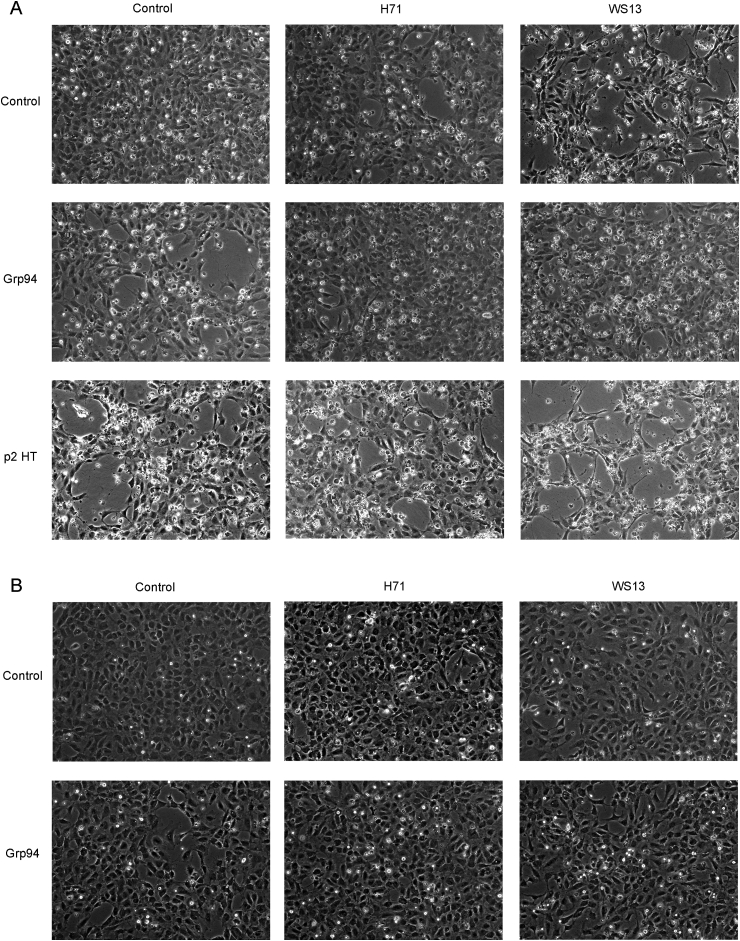
Both Grp94 and p2HT drastically changed cell morphology in absence of plasma, inducing the formation of numerous cavities and long cytoplasmic elongations of cells suggestive of a marked angiogenic-like transformation (Fig. 3A). PU-H71 (1 μM), but not so PU-WS13 (1 μM), reduced the alterations caused by Grp94, whereas both PU-inhibitors were apparently unable to significantly counteract the effects of p2HT. It was observed that the normal morphology of HUVECs was also partially subverted by PU-inhibitors alone, especially PU-WS13. The Grp94-dependent morphological alterations of HUVECs were much less visible in presence of plasma so that any antagonistic effect of the inhibitors was not detectable in this condition (Fig. 3B).
Fig. 3.
PU-inhibitors differently affect cell morphology in both absence and presence of Grp94 and native Grp94-IgG complexes. Cells were seeded at the concentration of 25 × 104 in triplicate wells (12-well plate) and incubated in both absence (A) and presence of 1% human plasma (B) with Grp94 and p2HT (100 ng/ml each) and PU-inhibitors (1 μM both). P2HT was only tested in absence of plasma (A). Representative images are shown of many others taken at the optical microscope (Leyca DMI 40008 equipped with DFC camera 480) in at least four experiments made on separate occasions. Magnification of 10 x.
3.3. PU-inhibitors activate PI3/Akt and VEGF pathways in HUVECs independently of Grp94 and p2HT but show distinct sensitivity to ATP
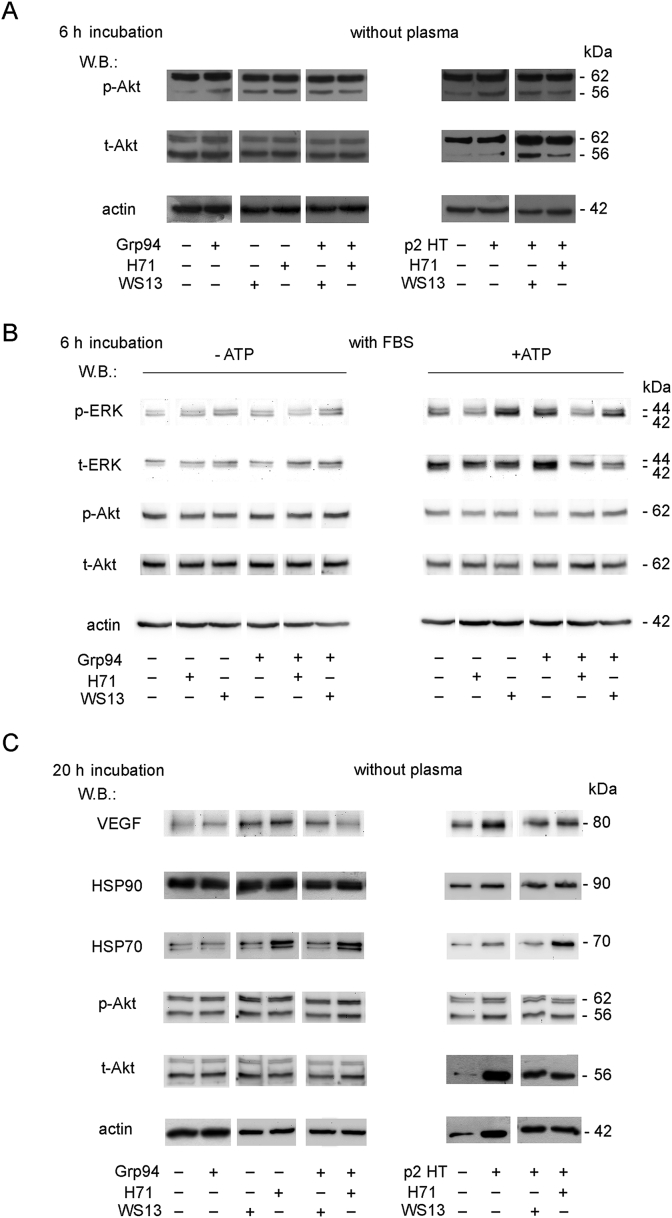
Inhibition of the angiogenic-like capacity of Grp94 can be explained by occupancy of the ATP-binding site by PU-inhibitors with consequent conformational modification of Grp94, so as to prevent its binding to the cell. However, since neither one of the PU-inhibitors, especially PU-WS13, appeared to antagonize the effects of Grp94 on both cell viability and morphology, suggesting instead an independent capacity of PU-inhibitors to affect cell metabolism, we investigated this aspect further by measuring the expression of proteins of the signaling pathways specifically involved in the process of growth and differentiation of HUVECs [27,28]. Proteins of both MEK-ERK1/2 and p38 pathways did not change with respect to the control for the addition of any substance at 6 h incubation in absence of plasma/serum, whereas a slight stimulation of the p-Akt was detected with both Grp94 and p2HT (Fig. 4A). PU-inhibitors appeared to partially inhibit the stimulation of p-Akt (the 56-kDa isozyme) by p2HT (Fig. 4A, right), at the same time stimulating on their own the expression of p-Akt (Fig. 4A, left). The stimulation of p-Akt by both Grp94 and PU-inhibitors was no longer visible when ATP was added to the medium (data not shown) and also when cells were cultured in presence of serum (Fig. 4B, left). In presence of serum, ATP reduced the expression of both p- and t-Akt while causing a significant stimulation of both p- and t-ERK1/2, independently of that induced by Grp94 and PU-WS13 (Fig. 4B, right). Both Grp94 and PU-WS13 appeared to enhance the stimulatory effect of ATP, whereas PU-H71 behaved differently and inhibited MEK-ERK1/2 activation by Grp94 (Fig. 4B, right). Opposite effects on Akt and MEK-ERK1/2 have already been observed to occur with ATP and attributed to the activation of the P2X7 receptor [29,30]. In particular, the significant depletion of the p-Akt has been associated with a potential killing activity of ATP in cancer cells [31]. Apparently, thus, ATP was responsible for reversing the PI3K-Akt stimulation by Grp94 and PU-inhibitors, while in presence of serum permitted to disclose the different capacity of PU-H71 and PU-WS13 to interfere with the MEK-ERK1/2 pathway independently of Grp94.
Fig. 4.
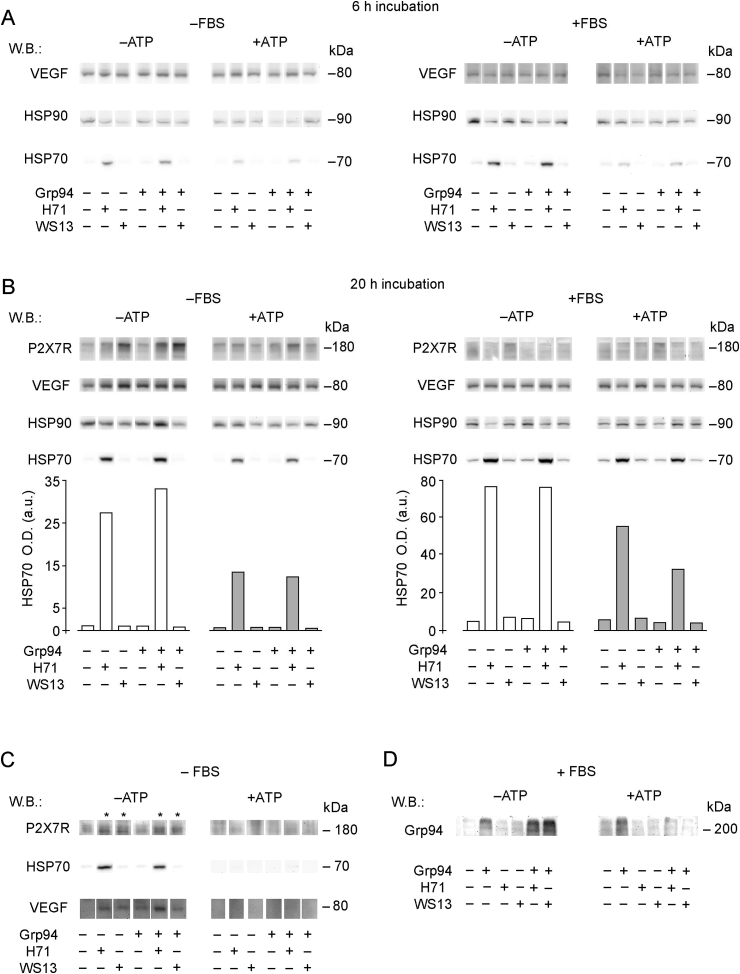
PU-inhibitors interfere with specific cell signaling pathways independently of Grp94 but dependently on ATP. Cells were cultured in duplicate wells as specified in Methods for obtaining and analyzing cell lysates. A. Western blotting for the indicated proteins was performed after 20 μg proteins of each sample were processed in SDS-PAGE (8.5% gel polyacrylamide) in reducing conditions. Cells were cultured for 6.0 h in absence of plasma and incubated with Grp94 and p2HT (100 ng/ml each) and PU-inhibitors (0.5 μM PU-H71 and 1 μM PU-WS13). B. Cells were cultured in presence of 1% FBS with and without 0.5 mM ATP. Conditions of Western blotting are as in A. Western blotting in absence and presence of ATP refer to two separate gels and membranes, each exposed to specific antibodies in sequence (from above) following stripping. C. Cells were cultured in absence of plasma with Grp94 and p2HT and PU-inhibitors as in A, and incubated for 20 h. Samples (20 μg) for measuring VEGF, HSP90 and HSP70 are loaded on a gel distinct from that for detecting p-Akt and t-Akt (samples loaded in reducing conditions). Molecular masses of reference on right of WB in each panel.
After 20 h incubation, the stimulation of PI3K-Akt was no longer detectable with Grp94 and PU-inhibitors alone, whereas still partially persisted with p2HT (Fig. 4C). The result is in accord with what already observed on HUVECs with in vitro-formed Grp94-IgG complexes which caused a prolonged activation of PI3K-Akt [32]. Since both HSP90 and HSP70 are involved in mediating the effects of Grp94 on HUVECs [22], and since VEGF is known to primarily contribute to the angiogenic transformation of endothelial cells, we also measured the expression of these proteins at the two incubation times. No stimulation was observed at 6 h in absence of plasma/serum (Fig. 5A, left), whereas at 20 h VEGF expression was slightly stimulated by Grp94 and to a higher extent by p2HT (Fig. 4C) and PU-inhibitors (Figs. 4C and 5B). Stimulation of VEGF by PU-inhibitors appeared to be independent of that sustained by Grp94 and p2HT. As expected, PU-H71 increased the expression of HSP70 [14] in both absence and presence of plasma, whereas no variation with respect to the control was noted for HSP90 (Figs. 4C and 5A, left). Results support the view that PU-inhibitors are able to modulate the PI3K-Akt and VEGF pathways in HUVECs independently of Grp94 and p2HT.
Fig. 5.
ATP antagonizes the effects of PU-inhibitors on HUVECs. HUVECs (25 × 104/well) were cultured as indicated in Methods in duplicate wells in absence and presence of 1% FBS, with and without ATP (0.5 mM), and incubated with Grp94 (100 ng/ml) and PU-inhibitors (250 nM) for 6.0 h (A) and 20 h (B). After each incubation period, cells were collected and lysed as specified in Methods, and 15 μg proteins (without reducing treatment) loaded on a 4–20% polyacrylamide gels for SDS-PAGE followed by Western blotting for testing, in sequence after stripping of the membrane, the expression of P2X7R, VEGF, HSP90 and HSP70. In panel B, graphs represent the intensity of HSP70-positive bands measured by densitometric analysis (GelPro Software) and expressed as arbitrary units of optical density (O.D.). A representative blotting of three others made on different occasions is shown. C. Cells were cultured at the density indicated above and incubated with both Grp94 and PU-inhibitors (as above) in absence of FBS for 20 h. Media were then collected, dialyzed, lyophilized and re-suspended in 50 μl of sample buffer. Fifteen μl of the protein solution (not submitted to reducing treatment) were loaded on a 4–20% gradient polyacrylamide gel for SDS-PAGE followed by Western blotting for the proteins listed in A and B. A representative blotting (for the proteins that only gave positive results) is shown of three others made on separate occasions. D. Cells were cultured as indicated above and incubated with Grp94 (100 ng/ml) and PU-inhibitors (250 nM) in presence of 1% FBS for 20 h. Media were collected, dialyzed, lyophilized and re-suspended in 80 μl of sample buffer. Fifteen μl of the protein solution were loaded on a 4–20% gradient polyacrylamide gel for SDS-PAGE followed by Western blotting for Grp94. A representative blotting is shown of two others made on separate occasions.
3.4. Both ATP and serum interfere with the activation of P2X7 receptor by PU-inhibitors
The observation that ATP modulates the effects of Grp94 and PU-inhibitors on PI3K-Akt and MEK-ERK1/2 in HUVECs led us to investigate this aspect in detail. We centered the attention on P2X7R that, for being one of the most important sub-types of ATP receptor, also present in endothelial cells [33,34], was the more probable target of PU-inhibitors. P2X7R was scarcely expressed in HUVECs at 6 h in any incubation condition, whereas at 20 h, in absence of serum and ATP, it was markedly stimulated by PU-inhibitors, mostly PU-WS13 (Fig. 5B, left). In non-reducing condition of samples in SDS-PAGE, the receptor was always found at molecular mass higher than that predicted for the full-length form of 60–70 kDa [35], suggesting either oligomeritation [36] or association of the receptor with other cell proteins [37]. Accordingly, after reducing treatment of samples, P2X7R migrated at the molecular mass of 65 kDa in SDS-PAGE (data not shown). It was apparent that the stimulation of VEGF by PU-inhibitors paralleled that of P2X7R, whereas this was not true for Grp94 that did not affect at all the P2X7R expression (Fig. 5B, left).
The results confirm the already observed relationship between P2X7R activation and release of VEGF [26,30], and corroborate the hypothesis that Grp94 and PU-inhibitors activate PI3K-Akt by targeting distinct upstream cell structures. It is known that P2X7R is present on cell membrane in complexes with other proteins responsible for coupling the activation of the receptor to a series of downstream signaling cascades [38]. It has also been demonstrated that both HSP70 and HSP90 can form complexes with the receptor, and HSP90 in particular is closely involved in the regulation of the receptor [37,39]. We measured the expression of these HSPs in cells cultured in both absence and presence of FBS and ATP and found that in neither condition Grp94 changed the expression of HSP90 and HSP70 that was instead stimulated by PU-H71 as a function of time of incubation and serum addition (Fig. 5A and B). Interestingly, in both absence and presence of serum, ATP antagonized the stimulation of HSP70, an effect that was still visible after 20 h incubation (Fig. 5B). It was observed that the capacity to stimulate P2X7R and VEGF by PU-inhibitors was lost in presence of ATP, as it was with serum regardless of ATP addition (Fig. 5B, right). Thus, both ATP and serum reversed the P2X7R stimulation by PU-inhibitors, whereas only ATP was apparently responsible for the antagonistic effect on the stimulation of HSP70 expression.
3.5. ATP antagonizes the PU-inhibitor-mediated secretion of P2X7R, VEGF and HSP70 from HUVECs
Considering the inhibitory effect of ATP on the stimulation by PU-inhibitors of P2X7R, VEGF and mostly HSP70 in cell lysates, it was of interest to explore whether similar antagonism could also occur on protein secretion. The measurement of protein expression in cell media was possible only for cells cultured in absence of serum since the bulk of albumin and IgG in serum (despite the low concentration of 1%) precluded the evaluation of any true difference in the pattern of protein secreted. At 6 h incubation, only weak expression of the proteins were visible in cell media, probably due to the insufficient secretion (data not shown), whereas at 20 h a significant positivity for P2X7R and VEGF were found with both PU-inhibitors, and also for HSP70 with PU-H71 only (Fig. 5C). ATP reversed this pattern and no protein was anymore detectable in the medium (Fig. 5C).
3.6. Inhibition of the Grp94-IgG complex formation by PU-inhibitors in a cellular setting crucially depends on ATP
Since the experiments aimed at testing the capacity of PU-inhibitors to prevent the formation of Grp94-IgG complexes in a cellular setting failed to address this issue – the morphological alterations induced by Grp94 on HUVECs were apparently reduced in presence of plasma/serum (Fig. 3B), so that any inhibitory effect by PU-inhibitors was not testable in this condition - we sought to address this issue more directly, also taking advantage of the above reported effects of ATP. Cells were thus incubated with Grp94 and PU-inhibitors in presence of FBS, with and without ATP, and the expression of Grp94 was measured in media. In this experiment, PU-inhibitors were used at the concentration of 250 nM that falls within the range of concentrations reported to yield half-maximal tumoricidal activity [6]. As expected, Grp94 formed high-molecular mass complexes with IgG present in FBS and the complex formation was not prevented by the addition of PU-inhibitors in absence of ATP (Fig. 5D), suggesting that in this condition binding of PU-inhibitors to the receptor on HUVECs was favored with respect to the ATP site in Grp94. Instead, in presence of ATP, PU-inhibitors caused a marked reduction in the complex formation as judged by disappearance of the bands at the highest molecular masses in WB (Fig. 5D). The result can be explained by considering that ATP has a higher affinity to P2X7R than PU-inhibitors that, for being prevented from binding to the cell receptor, are free to bind to Grp94 blocking Grp94 binding to IgG. The finding that Grp94-IgG complexes that form in FBS are not modified by PU-inhibitors in absence of ATP further confirms what already observed in experiments with p2HT, i.e., that PU-inhibitors cannot access Grp94 when it is stably complexed with IgG.
4. Discussion
The aim of our work was to test the possibility that PU-inhibitors could antagonize the effects driven on vascular cells by Grp94. In particular, since Grp94 following its extracellular liberation is found almost exclusively in complexes with IgG [24], we wanted to see whether PU-inhibitors, by binding to the ATP site in Grp94 could also prevent Grp94 binding to IgG. Considering the strong stability and deleterious effects driven on vascular cells by circulating Grp94-IgG complexes [21,23], prevention of the Grp94-IgG complex formation might represent a better therapeutic option. Here, we demonstrate that when tested in presence of human plasma, in a-cellular setting, PU-H71 and PU-WS13 actually block the formation of complexes of Grp94 with IgG by occupying the ATP site in Grp94 (Fig. 1), a result in line with previous observations showing that the binding of IgG takes place in the N-terminal portion of Grp94, nearby the ATP site [25]. We instead failed to demonstrate a similar antagonism when PU-inhibitors were tested on HUVECs, in presence of both Grp94 and native Grp94-IgG complexes purified from diabetic plasma (p2HT). Indeed, the experiments in which cells were cultured in presence of IgG (by adding either FBS or human plasma to cell cultures) – the condition used to test the capacity of PU-inhibitors to prevent the complex formation by Grp94 - did not permit to draw any firm conclusion, since, in presence of plasma or serum, the effects driven on cells by Grp94 were always less evident and inconstant (Fig. 2, Fig. 3). This can be explained by considering that besides binding to PU-inhibitors, Grp94 also binds to IgG, forming complexes that are however in concentrations not sufficient to drive significant effects on cells, so that the net result is less intense than that observed with Grp94 in absence of serum.
In experiments in which cells were instead cultured in absence of serum/plasma, a marked stimulation of cell growth and significant alterations of cell morphology were evident with both Grp94 and p2HT (Fig. 2, Fig. 3). PU-H71 caused a concentration-dependent reduction in the stimulatory effect of Grp94, as well as inhibition of the morphological alterations (Fig. 3A), whereas PU-WS13 was apparently much less effective in this respect. However, also PU-H71 did not reduce significantly the angiogenic-like stimulation of p2HT, a result consistent with the inability of PU-inhibitors to access the ATP site in Grp94 once this is stably linked to IgG [25] (Fig. 1B). The result of a different inhibitory capacity of the two inhibitors, PU-WS13 showing little or no ability to reverse the effects of Grp94 on cells even at the highest concentration and despite its higher specificity as Grp94 inhibitor [8], raised doubt on whether the observed effects could really be attributable to the shared mechanism of blocking the ATP site in Grp94. If this were the case, indeed, we would expect to observe overlapping or even higher inhibitory capacity by PU-WS13. It was thus considered the possibility that PU-inhibitors, besides blocking the ATP site in Grp94, could also display a similar mechanism directly on vascular cells, a conclusion confirmed in experiments in which PU-inhibitors were shown to sustain cell viability and induce morphological modifications in HUVECs similar to, although less intense than those provoked by Grp94 and p2HT (Fig. 2, Fig. 3). These results strongly suggest that the effects of PU-inhibitors were mediated by the same cellular pathway(s) involved in Grp94-and p2HT-driven effects, although the upstream membrane target should predictably be distinct. By investigating the cell signaling pathways commonly involved in mediating growth-stimulating and transforming effects on endothelial cells, we found that at short incubation time both Grp94 and p2HT activated PI3K-Akt, whereas after 20 h incubation only p2HT was still able to stimulate the expression of Akt together with that of VEGF (Fig. 4C), a finding consistent with the already observed capacity of native Grp94-IgG complexes to sustain prolonged and intense effects on endothelial cells [23]. Coherently with results of the morphological pictures, we noted that when PU-inhibitors were incubated with both Grp94 and p2HT, cell expression of p-Akt and t-Akt, as well as that of VEGF overlapped that measured with PU-inhibitors alone (Fig. 4A), to confirm the conclusion that PU-inhibitors stimulate the cell signaling pathways that lead to the endothelial cell transformation independently of Grp94 and p2HT. Taking into account the above results and also considering the structural and functional properties of PU-inhibitors, it was hypothesized that the likely cellular target of PU-inhibitors could be the P2X7 receptor, the main ATP-gated ion channel receptor [34], and that, similarly to the antagonism occurring with ATP at the binding site in Grp94, antagonism might also take place between PU-inhibitors and ATP at the cellular level where ATP displays a range of metabolic effects through the activation of this receptor in both physiological [33,34] and pathological conditions [30,40]. Although it has been reported that ATP has paracrine effects on vascular cells, inducing the growth and differentiation of endothelial cells [33,41], in our experiments ATP (0.5 mM) did not modify cell viability or morphology, nor did it significantly affect the effects of Grp94 on HUVECs (Fig. 2B). It was also apparent that ATP did not stimulate the expression of P2X7R (Fig. 5) whereas it was able to cause a reduction in the basal level of both p-Akt and t-Akt together with stimulation of p-ERK1/2 and t-ERK1/2 (Fig. 4B), both these effects being justified by the activation of P2X7R, as has also been demonstrated in other cell types [[29], [30], [31]]. The effects of ATP on both PI3K-Akt and MEK-ERK1/2 pathways also prevailed in presence of Grp94 and PU-inhibitors, since neither Grp94 nor PU-inhibitors per se gave any stimulation of MEK-ERK1/2 that instead appeared following the addition of ATP (Fig. 4B). In this respect, PU-H71 behaved differently with respect to PU-WS13 since in presence of ATP no stimulation of MEK-ERK1/2 was observed with PU-H71, a fact that pointed to a different mechanism of regulation of cell metabolism by the two inhibitors. The finding that also serum prevented the activation of PI3K-Akt might be explained by considering that serum in itself is able to sufficiently stimulate MEK-ERK1/2 as to negatively regulate PI3K-Akt. The cross-talk between the two pathways is reported to follow an inverse regulation so that activation of one pathway associates with inhibition of the other [42].
Since the antagonism exerted by ATP on activation of PI3K-Akt by Grp94 and PU-inhibitors implied the possible involvement of P2X7R, we addressed this issue in separate experiments and verified that PU-inhibitors, but not Grp94, increased the expression of P2X7R in absence of serum, an effect completely antagonized by ATP (Fig. 5B). Besides supporting the conclusion suggested by experiments on cell viability (Fig. 2), i.e., that the effects of Grp94 on Akt and VEGF are mediated by an upstream cell membrane signal distinct from that targeted by PU-inhibitors, results clearly indicated that the effects of PU-inhibitors on HUVECs involved the purinergic pathway. Intriguingly, however, the activation of this pathway did not appear to directly involve the P2X7 receptor whose activation by ATP is known to cause phosphorylation of MEK-ERK1/2 [30] while causing depletion of phosphorylated Akt [31]. Although both these effects have been documented to occur in cancer cells and no such detailed information is so far available in vascular endothelial cells, our results nevertheless demonstrated that PU-inhibitors do not share with ATP the same mechanism of activation of the purinergic pathway since the increased expression of P2X7R by PU-inhibitors primarily appeared to depend on activation of PI3K-Akt. Associated with this mechanism was the increased expression of both VEGF, induced by both PU-inhibitors, and HSP70, exclusively induced by PU-H71. Inhibition of HSP90 by pan-HSP90 inhibitors like PU-H71 is known to increase the expression of HSP70 in cancer cells [3,8], an effect that counteracts the anti-tumor efficacy of HSP90 inhibitors [43,44]. We observed that the increase in HSP70 expression by PU-H71 was a time-dependent and serum-independent effect (Fig. 5A and B). Interestingly, ATP was able to inhibit the stimulation of HSP70 in both absence and presence of serum, this effect being maximal at short incubation time but still significant even after 20 h incubation (Fig. 5A and B) when a reduction in ATP concentration is expected to occur due to ATP degradation. Antagonism by ATP of the PU-inhibitor effects on the purinergic pathway was also visible on secretion, since the time-dependent secretion of both P2X7R and VEGF and of HSP70 (for PU-H71 only) was abolished by ATP (Fig. 5C). An extra-cellular liberation of P2X7R has been described to occur following activation of the purinergic pathway that causes exocytosis of microvesicles containing, among others, also the receptor itself [45].
Since our results collectively indicated that antagonism between PU-inhibitors and ATP occurred almost exclusively at the cellular level, being unobservable any competition at the ATP site in Grp94 (Figs. 2 and 5), we took advantage of this antagonism to see whether ATP could influence the capacity of PU-inhibitors to block Grp94 so as to prevent binding to IgG. Thus, by measuring the expression of Grp94-IgG complexes in media of cells cultured with serum in presence of both Grp94 and PU-inhibitors, it was observed that in absence of ATP PU-inhibitors did not prevent binding of Grp94 to IgG so that complexes were visible in Western blotting (Fig. 5D). This result was in line with what observed on cell morphology, i.e. that also in presence of Grp94 the effects on cells were those driven by PU-inhibitors, thus implying that in these experimental conditions PU-inhibitors show a higher binding affinity to the cell target than the ATP site on Grp94. This conclusion was further supported by results obtained with the addition of ATP, since in this case PU-inhibitors were able to antagonize the formation of Grp94-IgG complexes (Fig. 5D): thus, ATP by competing with PU-inhibitors at the binding site on cell, enabled PU-inhibitors to be available to bind and block Grp94.
In conclusion, our work has disclosed the so far unknown property of PU-inhibitors to target the ATP receptor P2X7 and the purinergic pathway in HUVECs with a mechanism different from that displayed by the natural ligand ATP. Binding of PU-inhibitors to the cell membrane is such as to prevent occupancy of the ATP site in Grp94 so that effects on cells in presence of Grp94 are those displayed by PU-inhibitors alone. In this respect, PU-inhibitors appear to partially mitigate the intense angiogenic-like effect of Grp94. At the same time, however, binding of PU-inhibitors to cell permits unblocked Grp94 to bind to IgG thus forming Grp94-IgG complexes. Our results demonstrate that ATP, by binding to P2X7R on HUVECs, can reverse the effects of PU-inhibitors, including the unwanted stimulation of HSP70 by PU-H71, leaving PU-inhibitors free to block Grp94 binding to IgG. This last mechanism might be exploited to enhance the therapeutic efficacy of PU-inhibitors to prevent the pathogenic effects of circulating Grp94-IgG complexes.
Acknowledgments
We would like to thank Dr. G. Chiosis of the Memorial Sloan Kettering Cancer Center (NY, USA) for kindly providing us with PU-inhibitors.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100661.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Workman P., Burrows F., Neckers L., Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogenic addiction and tumor stress. Ann. N. Y. Acad. Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 2.Breining M., Caldas-Lopes E., Goeppert B., Malz M., Rieker R., Bergmann F., Scirmacher P., Mayer M., Chiosis G., Kern M.A. Targeting heat shock protein 90 with non-quinone inhibitors: a novel chemotherapeutic approach in human hepatocellular carcinoma. Hepatology. 2009;50:102–112. doi: 10.1002/hep.22912. [DOI] [PubMed] [Google Scholar]
- 3.Caldas-Lopes E., Cerchietti L., Ahn J.H., Clement C.C., Robles A.I., Rodina A., Moulick K., Taldone T., Gozman A., Guo Y., Wu N., de Stanchina E., White J., Gross S.S., Ma Y., Varticovski L., Melnick H., Chiosis G. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple negative breast cancer models. Proc. Natl. Acad. Sci. U.S.A. 2009;106(20):8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee A.S. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat. Rev. Canc. 2014;14:263–276. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okawa Y., Hideshima T., Steed P., Vallet S., Hall S., Kuang K., Rice J., Barabasz A., Foley B., Ikeda H., Raje N., Kilziltepe T., Yasui H., Enatsu S., Anderson K.C. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood. 2009;113(4):846–855. doi: 10.1182/blood-2008-04-151928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usmani S.Z., Bona R.D., Chiosis G., Li Z. The anti-myeloma activity of a novel purine scaffold HSP90 inhibitor PU-H71 is via inhibition of both HSP90A and HSp90B1. J. Hematol. Oncol. 2010;3:40. doi: 10.1186/1756-8722-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitesell L., Lindquist S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Canc. 2005;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 8.Patel P.D., Yan P., Seidler P.M., Patel H.J., Sun W., Yang C., Que N.S., Taldone T., Finotti P., Stephani R.A., Gewirth D.T., Chiosis G. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol. 2013;9(11):677–684. doi: 10.1038/nchembio.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taldone T., Gozman A., Maharaj R., Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr. Opin. Pharmacol. 2008;8(4):370–374. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Que N.L.S., Crowley V.M., Duerfeldt A.S., Zhao J., Kent C.N., Blagg B.S.J., Gewirth D.T. Structure based design of a Grp94-selective inhibitor: exploiting a key residue in Grp94 to optimize paralog-selective binding. J. Med. Chem. 2018;61(7):2793–2805. doi: 10.1021/acs.jmedchem.7b01608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jhaveri K., Taldone T., Modi S., Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancer. Biochem. Biophys. Acta. 2012;1823(3):742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travers J., Sharp S., Workman P. HSP90 inhibition: two-pronged exploitation of cancer dependencies, Drug Discov. Today Off. 2012;17(5–6):242–252. doi: 10.1016/j.drudis.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Guo A., Lu P., Lee J., Zhen C., Chiosis G., Wang Y.L. HSP90 stabilizes B-cell receptor kinases in a multi-client interactome: PU-H71 induces CLL apoptosis in a cytoprotective microenvironment. Oncogene. 2017;36(24):3441–3449. doi: 10.1038/onc.2016.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duerfeldt A.S., Peterson L.B., Maynard J.C., Ng C.L., Eletto D., Ostrovsky O., Shinogle H., Moore D.S., Argon Y., Nicchitta C.V., Blagg B.S. Development of a Grp94 inhibitor. J. Am. Chem. Soc. 2012;134(23):9796–9804. doi: 10.1021/ja303477g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel H.J., Patel P.D., Ochiana S.O., Yan P., Sun W., Patel M.R., Shah S.K., Tramentozzi E., Brooks J., Bolaender A., Shrestha L., Stephani R., Finotti P., Leifer C., Li Z., Gewirth D.T., Taldone T., Chiosis G. Structure-activity relationship in a purine-scaffold compound series with selectivity for the endoplasmic reticulum Hsp90 paralog Grp94. J. Med. Chem. 2015;58(9) doi: 10.1021/acs.jmedchem.5b00197. 3922-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha L., Bolaender A., Patel H.J., Taldone T. Heat Shock protein (HSP) drug discovery and development: targeting Heat Shock Proteins in disease. Curr. Top. Med. Chem. 2016;16(25):2753–2764. doi: 10.2174/1568026616666160413141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodina A., Wang T., Yan P., Gomes E.D., Dunphy M.P., Pillarsetty N., Koren J., Gerecitano J.F., Taldone T., Zong H., Caldas-Lopes E., Alpaugh M., Corben A., Riolo M., Beattie B., Pressl C., Peter R.I., Xu C., Trondl R., Patel H.J., Shimizu F., Bolaender A., Yang C., Panchal P., Farooq M.F., Kishinevsky S., Modi S., Lin O., Chu F., Patil S., Erdjument-Bromage H., Zanzonico P., Hudis C., Studer L., Roboz G.J., Cesarman E., Cerchietti L., Levine R., Melnick A., Larson S.M., Lewis J.S., Guzman M.L., Chiosis G. The epichaperome is an integrated chaperome network that facilitates tumor survival. Nature. 2016;538(7625):397–401. doi: 10.1038/nature19807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C.Y., Batzorig U., Cheng W.L., Huang M.T., Chen W.Y., Wei P.L., Chang Y.J. Glucose-regulated protein 94 mediates cancer progression via AKT and eNOS in hepatocellular carcinoma. Tumor Biol. 2015;37(4):4295–4304. doi: 10.1007/s13277-015-4254-9. [DOI] [PubMed] [Google Scholar]
- 19.Wu B.X., Hong F., Zhang Y., Ansa-Addo E., Li Z. GRP94/gp96 in cancer: biology, structure, immunology, and drug development. Adv. Cancer Res. 2016;129:165–190. doi: 10.1016/bs.acr.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Pagetta A., Folda A., Brunati A.M., Finotti P. Identification and purification from the plasma of type 1 diabetic subjects of a proteolytically active Grp94. Diabetologia. 2003;46:996–1006. doi: 10.1007/s00125-003-1133-5. [DOI] [PubMed] [Google Scholar]
- 21.Roveri A., Zaccarin M., Pagetta A., Tramentozzi E., Finotti P. Proteomic investigation on Grp94-IgG complexes circulating in plasma of Type 1 diabetic subjects. J. Diabetes Res. 2015 doi: 10.1155/2015/815839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tramentozzi E., Montopoli M., Orso G., Pagetta A., Caparrotta L., Frasson M., Brunati A.M., Finotti P. Stable complexes formed by Grp94 with human IgG promoting angiogenic differentiation of HUVECs by a cytokine–like mechanism. Mol. Immunol. 2008;45:3639–3648. doi: 10.1016/j.molimm.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Tramentozzi E., Pagetta A., Frasson M., Brunati A.M., Montopoli M., Finotti P. Angiogenic transforming capacity of IgG purified from plasma of type 1 diabetic subjects. J. Cell Mol. Med. 2009;13:1336–1347. doi: 10.1111/j.1582-4934.2008.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tramentozzi E., Ruli E., Angriman I., Bardini R., Campora M., Guzzardo V., Zamarchi R., Rossi E., Rugge M., Finotti P. Grp94 in complexes with IgG is a soluble diagnostic marker of gastrointestinal tumors and displays immune-stimulating activity on peripheral blood immune cells. Oncotarget. 2016;7(45):72923–72940. doi: 10.18632/oncotarget.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagetta A., Tramentozzi E., Tibaldi E., Cendron L., Zanotti G., Brunati A.M., Vitadello M., Gorza L., Finotti P. Structural insights into complexes of Glucose-regulated Protein94 (Grp94) with human immunoglobulin G. Relevance for Grp94-IgG complexes that form in vivo in pathological conditions. PLoS One. 2014 doi: 10.1371/journal.pone.0086198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Virgilio F. Purines, purinergic receptors and cancer. Cancer Res. 2012;72:5441–5447. doi: 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- 27.Ebisuya M., Kondoh K., Nishida E. The duration, magnitude and compartmentalization of ERK MAP kines activity: mechanisms for providing signaling specificity. J. Cell Sci. 2005;118(Pt 14):2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 28.Somanath P.R., Razorenova O.V., Chen J., Byzova T.V. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5(5):512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monteiro da Cruz C., Marquez Ventura A.L., Scachter J., Costa-Junior H.M., da Silva Souza H.A., Ramos Gomez F., Coutinho-Silva R., Ojcius D.M., Muanis Persechini P. Br. J. Pharmacol. 2006;147:324–334. doi: 10.1038/sj.bjp.0706559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Virgilio F., Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36(3):293–303. doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bian S., Sun X., Bai A., Zhang C., Li L., Enjyoji K., Junger W.G., Robson S.C., Wu Y. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling PIs to mediate tumor cell death. PLoS One. 2013;8(4):e60184. doi: 10.1371/journal.pone.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tramentozzi E., Tibaldi E., Brunati A.M., Pagetta A., Finotti P. Crucial role of HSP90 in the Akt-dependent promotion of angiogenic-like effect of glucose-regulated protein94 (Grp94)-IgG complexes. J. Cell Mol. Med. 2011;15(12):2768–2780. doi: 10.1111/j.1582-4934.2011.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohman A.W., Billaud M., Isakson B.E. Mechanisms of ATP release and signaling in the blood vessel wall. Cardiovasc. Res. 2012;95:269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohman J., Erlinge D. The touching story of purinergic signaling in epithelial and endothelial cells. Purinergic Signal. 2012;8:599–608. doi: 10.1007/s11302-012-9316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Killeen M.E., Ferris L., Kupetsky E.A., Falo L., Jr., Mathers A.R. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J. Immunol. 2013;190:4324–4336. doi: 10.4049/jimmunol.1202045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.North R.A., Jarvis M.F. P2X receptors as drug targets. Mol. Pharmacol. 2013;83:759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalo U., Jones S., Roberts J.A., Mahaut-Smith M.P., Evans R.J. Heat shock protein 90 inhibitors reduce trafficking of ATP-gated P2X1 receptors and human platelet responsiveness. J. Biol. Chem. 2012;287:32747–32754. doi: 10.1074/jbc.M112.376566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M., Jiang L.H., Wilson H.L., North R.A., Surprenant A. Proteomic and functional evidence for a P2X7 receptor signaling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adinolfi E., Kim M., Young M.T., Di Virgilio F., Surprenant A. Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J. Biol. Chem. 2003;278:37344–37351. doi: 10.1074/jbc.M301508200. [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Villafuertes R., Garcia-Huerta P., Diaz-Hernandez J.I., Mira-Portugal M.T. PI3K/Akt signaling pathway triggers P2X7 receptor expression as a pro-survival factor of neuroblastoma cells under limiting growth conditions. Sci. Rep. 2015;5:8417. doi: 10.1038/srep18417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler. Thromb. Vasc. Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 42.Sunayama J., Matsuda K.I., Sato A., Tachibana K., Suzuki K., Narita Y., Shibui S., Sakurada K., Kayama T., Tomiyama A., Kitanaka C. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cell. 2010;28(11):1930–1939. doi: 10.1002/stem.521. [DOI] [PubMed] [Google Scholar]
- 43.Modi S., Stopeck A., Linden H., Solit D., Chandarlapaty S., Rosen N., D'Andrea G., Dickler M., Moynahan M., Sugarman M.E., Ma W., Patil S., Norton L., Hannah A.L., Hudis C. HSP90 inhibition is effective in breast cancer: a phase II trial of tenespimycin (17-AAG) plus trastuzumab. Clin. Cancer Res. 2011;17(15):5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 44.Graner M.W. HSP90 and immune modulation in cancer. Adv. Cancer Res. 2016;129:191–224. doi: 10.1016/bs.acr.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Qu Y., Dubyak G.R. P2X receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. 2009;5:163–173. doi: 10.1007/s11302-009-9132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.