Table 1.
Optimization of the Selective Cleavage of C(sp3)-F Bonds in Aliphatic gem-Difluoroalkanes
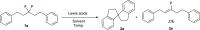 | ||||||
|---|---|---|---|---|---|---|
| Entry | Lewis Acids (Equiv.) | Solvent (0.1M) | T (°C) | t (h) | Yields (%) |
|
| 2a | 3aa | |||||
| 1 | BF3·OEt2 (2.2) | CH2Cl2 | RT | 30 | NR | NR |
| 2 | B(C6F5)3 (2.2) | CH2Cl2 | RT | 30 | 85 | 0 |
| 3 | B(C6F5)3 (0.2) | CH2Cl2 | RT | 30 | Trace | 0 |
| 4 | B(C6F5)3 (0.2) | (CF3)2CHOH | RT | 17 | 28 | 0 |
| 5 | B(C6F5)3 (0.2) | (CF3)2CHOH | 50 | 2 | 75 | 0 |
| 6 | B(C6F5)3 (0.1) | (CF3)2CHOH | 50 | 20 | 16 | 0 |
| 7 | B(C6F5)3 (0.2) | (CF3)2CHOHb | 50 | 2 | 27 | 0 |
| 8 | B(C6F5)3 (0.2) | (CF3)2CHOHc | 50 | 2 | 84 | 0 |
| 9 | B(C6F5)3 (0.2) | (CF3)2CHOHc H2O (2.2 equiv.) |
50 | 2 | 0d | 0d |
| 10 | B(C6F5)3 (0.2) | iPrOHc | 50 | 2 | NR | NR |
| 11 | B(C6F5)3 (0.2) | CF3CH2OHc | 50 | 2 | NR | NR |
| 12 | B(C6F5)3 (0.2) | (CF3)2PhOHc | 50 | 2 | NR | NR |
| 13 | – | (CF3)2CHOHc | 50 | 2 | NR | NR |
| 14 | B(C6F5)3 (0.2) | o-C6H4Cl2 | 160 | 3 | 0 | 64 |
| 15 | B(C6F5)3 (0.1) | o-C6H4Cl2 | 160 | 3 | 0 | 30 |
| 16 | – | o-C6H4Cl2 | 160 | 3 | NR | NR |
| 17 | B(C6F5)3 (0.2) | o-C6H4Cl2 | 220 | 3 | 0 | 81e |
| 18 | B(C6F5)3 (0.2) | p-C6H4F2 | reflux | 3 | 0 | 75 |
| 19 | B(C6F5)3 (0.2) | p-C6H4F2 | reflux | 24 | 0 | 87f |
RT, room temperature; NR, no reaction.
Determined by19F NMR analysis using PhCF3 as the internal standard.
(CF3)2CHOH was used as purchased without any precaution to exclude moisture. The hydrolysis product 1,5-diphenylpentan-3-one was observed as the major product.
Concentration: 0.05 M.
The hydrolysis product 1,5-diphenylpentan-3-one was obtained in quantitative yield after 12 h at 50°C.
Z/E = 7.3:1.
Z/E = 7.1:1.
