Table 2.
Control Experiments to Probe Reaction Mechanism
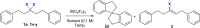 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Xa | Lewis Acids (Equiv.) | Solvent (0.1M) | T (oC) | t (h) | Yields (%) |
|
| 2a | 3ab | ||||||
| 1 | F | B(C6F5)3 (2.2) | CH2Cl2 | RT | 30 | 85 | 0 |
| 2 | 1v (C=O) | B(C6F5)3 (2.2) | CH2Cl2 | RT | 30 | NR | NR |
| 3 | MeO | B(C6F5)3 (2.2) | CH2Cl2 | RT | 30 | NDc | NDc |
| 4 | Cl | B(C6F5)3 (2.2) | CH2Cl2 | RT | 30 | NR | NR |
| 5 | Br | B(C6F5)3 (2.2) | CH2Cl2 | RT | 30 | 0 | 29 |
| 6 | F | B(C6F5)3 (0.2) | (CF3)2CHOHd | 50 | 2 | 84 | 0 |
| 7 | 1v (C=O) | B(C6F5)3 (0.2) | (CF3)2CHOHd | 50 | 2 | NR | NR |
| 8 | MeO | B(C6F5)3 (0.2) | (CF3)2CHOHd | 50 | 2 | NR | NR |
| 9 | Cl | B(C6F5)3 (0.2) | (CF3)2CHOHd | 50 | 2 | 0 | 63 |
| 10 | Cl | – | (CF3)2CHOHd | 50 | 2 | 0 | 61 |
| 11 | Br | B(C6F5)3 (0.2) | (CF3)2CHOHd | 50 | 2 | 0 | 81 |
| 12 | Br | – | (CF3)2CHOHd | 50 | 2 | 0 | 86 |
| 13 | F | B(C6F5)3 (0.2) | p-C6H4F2 | reflux | 24 | 0 | 87 |
| 14 | MeO | B(C6F5)3 (0.2) | p-C6H4F2 | reflux | 24 | 0 | 0 |
| 15 | Cl | B(C6F5)3 (0.2) | p-C6H4F2 | reflux | 24 | 0 | Trace |
| 16 | Br | B(C6F5)3 (0.2) | p-C6H4F2 | reflux | 24 | 0 | 9 |
NR, no reaction; RT, room temperature; ND, not detected.
Substrates: 1,5-diphenylpentan-3-one (1v), (3,3-dimethoxypentane-1,5-diyl)dibenzene (1w), (3,3-dichloropentane-1,5-diyl)dibenzene (1x), and (3,3-dibromopentane-1,5-diyl)dibenzene (1y).
NMR yields.
Complex mixture.
Concentration (0.05 M).
