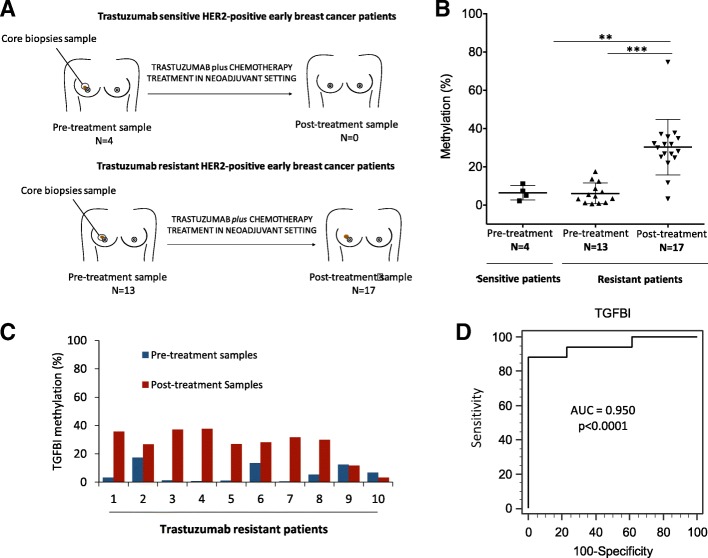
Fig. 4.
TGFBI promoter hypermethylation in HER2+ breast cancer patients with sensitivity and resistance to trastuzumab. a Schematic representation of selected patient samples. TGFBI methylation levels were evaluated in tumor samples of 24 HER2+ breast cancer samples. From this cohort, after trastuzumab plus chemotherapy in neoadjuvant regimen, 20 patients developed partial or no response and 4 patients presented complete treatment response. Of the 20 patients with non-response, 10 patients had pre-treatment and post-treatment samples, 3 patients with pre-treatment only samples, and 7 with post-treatment only samples (Top). The 4 patients with complete response to treatment only had pre-treatment samples (Bottom). b TGFBI methylation of 3 consecutive CpG sites in the 5′-end promoter CpG island in HER2+ breast cancer treated with trastuzumab analyzed by bisulfite pyrosequencing. The central solid line indicates the median and the limits of the vertical lines show the upper and lower percentiles. c TGFBI methylation of 3 consecutive CpG sites in the 5′-end promoter CpG island in resistant patients with paired pre- and post-treatment samples analyzed by bisulfite pyrosequencing. d Diagnostic accuracy of TGFBI hypermethylation for resistant HER2+ breast cancer samples. ROC analysis was applied to the TGFBI methylation levels analyzed by pyrosequencing for trastuzumab-resistant samples (pre- and post-treatment samples). Area under the curve (AUC) was 0.9502 (p < 0.0001). TGFBI showed great potential for monitoring trastuzumab response in HER2+ breast cancer patients. Significance of Mann-Whitney U test is indicated as ***p < 0.001; **p < 0.01