Abstract
Endometrial stem cells are located in the basal layer of the endometrium, and they are responsible for the cyclic regeneration of the uterus during the menstrual cycle. Recent studies have revealed that recurrent pregnancy loss is associated with an age-related stem cell deficiency in the endometrium. Therefore, intensive study of endometrial stem cell aging may provide new insights for preventing recurrent pregnancy loss. Sonic hedgehog (SHH) signaling has been identified as a morphogen during the embryonic development processes. In addition to this canonical function, we found that the age-associated decline in regenerative potential in the endometrium may be due to decreased SHH-signaling integrity in local stem cells with aging. Importantly, the current study also showed that SHH activity clearly declines with aging both in vitro and in vivo, and exogenous SHH treatment significantly alleviates various aging-associated declines in multiple endometrial stem cell functions, suggesting that SHH may act as an endogenous anti-aging factor in human endometrial stem cells. Moreover, we found that stem cell senescence may enhance SERPINB2 expression, which in turn mediates the effect of SHH on alleviating senescence-induced endometrial stem cell dysfunctions, suggesting that SERPINB2 is a master regulator of SHH signaling during the aging process.
Keywords: stem cells, aging, sonic hedge, SERPINB2
Graphical Abstract
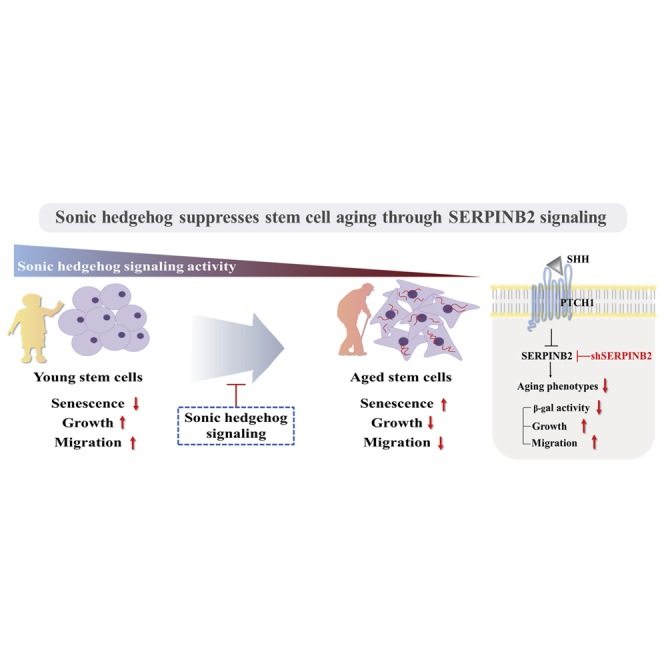
The age-associated decline in regenerative potential in the endometrium may be due to decreased SHH-signaling integrity in local stem cells with aging. In addition to its canonical function, SHH significantly alleviates various aging-associated declines in multiple endometrial stem cell functions, via SERPINB2 signaling as an endogenous anti-aging factor.
Introduction
Successful implantation and subsequent pregnancy primarily depend on dynamic interactions between the endometrium (inner lining of the uterus) and the implanting embryo.1 The endometrium undergoes approximately 500 cycles of growth, differentiation, shedding, and regeneration during a woman’s reproductive year in a tightly regulated manner.2 This extraordinary regenerative capacity of the endometrium is absolutely required for successful reproduction. Previous studies have revealed that the endometrium undergoes distinct age-associated physiologic and morphologic alterations, which are considered responsible for the significantly decreased reproductive potential with aging.3
Adult stem cells are generally characterized by their capacity to remain undifferentiated, renew themselves, and differentiate into a variety of cell types within a tissue.4 This unique regenerative capability makes aging-related cellular dysfunctions of local stem cells potentially more impactful than dysfunctions of other differentiated cell types.5 Like many other dynamically regenerating tissues, resident endometrial stem cells in the basal layer are responsible for this cyclic regeneration of the endometrium and uterine repair.6, 7 Importantly, a recent study revealed that endometrial stem cell dysfunction impairs the cyclic regeneration of the endometrium and subsequently decreases pregnancy and conception rates.8 Consistently, the cellular senescence of endometrial stem cells triggers chronic inflammation, which is a hallmark of aging9 and is subsequently associated with increased susceptibility to recurrent pregnancy loss.10 Therefore, intensive study of cellular senescence of endometrial stem cells may provide new insights into the mechanisms behind miscarriage and pave the way for successful reproduction.
Sonic hedgehog (SHH) signaling was originally identified as a morphogen that acts in a wide variety of patterning processes during embryonic limb development.11, 12 SHH binds to 12-transmembrane glycoprotein Patched1 (PTCH1), the principal receptor for SHH, which suppresses the activity of the 7-pass G-protein- coupled protein Smoothened (SMO).13 Subsequently, activated SMO releases glioma (GLI)-associated transcription factors from cytoplasmic sequestration, which in turn results in the nuclear localization of GLI to regulate various gene expressions.14 Interestingly, in addition to this canonical function, particular attention has been devoted to the noncanonical functions of SHH signaling in various aging-related diseases, such as neurodegenerative diseases15 and atherosclerosis,16 as its signaling integrity declines during the development of these age-associated chronic diseases. These findings suggest that SHH might act as an age-associated factor in addition to its canonical function. However, the noncanonical anti-aging effects of SHH signaling and its underlying molecular mechanisms in stem cells remain ill defined.
In this study, we hypothesized that SHH signaling could essentially act as an antagonist of endometrial stem cell loss and dysfunction in the aging process. Indeed, we showed for the first time that SHH expression was significantly decreased in multiple tissues, including the uterus, with aging. Importantly, exogenous SHH treatment markedly alleviated aging-associated declines in multiple endometrial stem cell functions, such as senescence-associated β-galactosidase activity, proliferation, and migration. We subsequently explored the molecular mechanism underlying the anti-aging effects of SHH on various endometrial stem cell functions. Interestingly, SHH significantly suppressed the expression of SERPINB2, which is also known as plasminogen activator inhibitor type 2 (PAI-2). Thus far, it has been shown that SERPINB2 expression is markedly enhanced in response to various differentiating agents in multiple cell types, such as keratinocytes,17 leukemia cells,18, 19 and mononuclear cells,20 suggesting that SERPINB2 could be involved in the aging process as a downstream target of SHH signaling. Importantly, the present study showed that downregulation of SERPINB2 with specific small hairpin RNA (shRNA) significantly attenuates the SHH-induced alleviating effects on stem cell aging. Taken together, these findings suggest that, in addition to its previously reported canonical activities, SHH activity clearly declines with aging as an endogenous anti-aging factor and subsequently alleviates age-related endometrial stem cell loss and dysfunction by suppressing the expression of SERPINB2.
Results
SHH Alleviates Various Aging-Associated Endometrial Stem Cell Dysfunctions In Vitro
Recently, Kovina et al.21 isolated and characterized menstrual blood-derived human endometrial stem cells that retained their ability to differentiate into multiple lineages. The isolation and characterization of endometrial stem cells from human endometrium were performed using a modification of their procedures. We first isolated endometrial stem cells from human endometrial tissues (Figure S1A), and then we characterized their biological properties using various stem cell surface markers, including CD34, CD44, CD45, CD73, CD105, CD140b, CD146, and W5C5 (Figure S1B). Four positive surface markers were mostly expressed (CD44, CD73, CD105, and CD140b), while a small percentage of some positive marker- (CD146 and W5C5) positive cells was detected in the whole cell population. Therefore, it is possible that our endometrial stem cells were a mixed-cell population consisting of at least two types of cells. Their transdifferentiation potential into multiple lineages was evaluated by inducing osteogenic and adipogenic differentiation (Figure S1C).
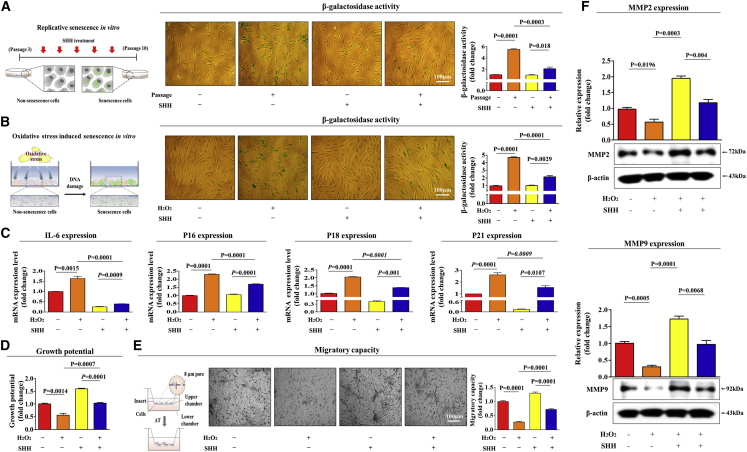
Serial passaging (replicative senescence) in culture or with oxidative stress exposure has been widely used to investigate the mechanisms of cellular aging in vitro.22, 23 The most distinctive measurable feature of both replicative and oxidative stress-induced senescence is the presence of a senescence-associated β-galactosidase (SA-β-Gal) enzymatic activity.24 Therefore, to investigate whether SHH treatment alleviates replicative senescence in vitro, endometrial stem cells were continuously subcultured with or without SHH treatment, as described in Figure 1A. The treatment concentration of SHH was assessed based on our two previous articles that revealed the stimulating effects of SHH signaling on the various stem cell functions.25, 26 Importantly, SHH significantly attenuated replicative senescence-induced SA-β-Gal activity (Figure 1A).
Figure 1.
SHH Attenuates Various Senescence-Associated Phenotypes in Endometrial Stem Cells In Vitro
Replicative and oxidative stress-mediated senescence were induced by continuous subculture until passage 10 and 700 nM hydrogen peroxide (H2O2) exposure for 1 h, respectively. Endometrial stem cells were continuously subcultured until passage 10 with or without SHH (4 μM) treatment. (A) The effects of SHH on stem cell aging in vitro were evaluated by measuring senescence-associated β-galactosidase (SA-β-Gal) enzymatic activity. (B) Endometrial stem cells were pretreated with 700 nM hydrogen peroxide (H2O2) for 1 h prior to treatment with 4 μM SHH for 48 h, and the changes in stem cell aging were determined by measuring SA-β-Gal activity. (C) The ability of SHH to attenuate oxidative stress-induced senescence marker expression (IL-6, p16, p18, and p21) was determined by real-time PCR. (D) Endometrial stem cells were pretreated with 700 nM hydrogen peroxide (H2O2) for 1 h prior to treatment with 4 μM SHH for 72 h, and the changes in cell viability were determined by an MTT assay. Stem cell viability (%) was calculated as a percent of the vehicle control. Changes in migratory capacity were measured via transwell assay (E) and western blotting for MMP-2 and MMP-9 (F). β-actin was used as an internal control. The results represent the means ± SD from three independent experiments.
To further determine the anti-aging effects of SHH on oxidative stress-induced senescence, endometrial stem cells were also exposed to hydrogen peroxide (H2O2) with or without SHH treatment, as shown in Figure 1B. Consistently, SHH markedly attenuated oxidative stress-induced SA-β-Gal activity (Figure 1B). We also evaluated whether H2O2 treatment actually induces apoptosis in endometrial stem cells by measuring apoptotic DNA fragmentation and caspase 3 activities. Interestingly, H2O2 treatment increased pro-apoptotic caspase 3 activity and subsequent DNA fragmentation (Figures S2A and S2B). Apart from SA-β-Gal activity, elevated expression levels of secreted or cytoplasmic proteins, such as interleukin (IL)-6, p16, p18, and p21, have been used as surrogate markers of cellular senescence in vitro.27 Both replicative and oxidative stress-induced senescence commonly activate the same senescence-associated phenotypes, such as SA-β-Gal activity (Figure S3A) and the expression of these intracellular or secreted aging markers (Figure S3B). Thus, we hereafter used oxidative stress-induced senescence as an in vitro model for endometrial stem cell aging in our experiments.
Importantly, the oxidative stress-induced expression of these senescence-associated markers was significantly attenuated by SHH treatment (Figure 1C). We conducted the additional set of experiments to further confirm the alleviating effects of SHH on oxidative stress-induced senescence with additional aging markers, such as RB1 and P14ARF. Consistently, oxidative stress-induced expression of these senescence-associated markers was significantly attenuated by SHH treatment (Figures S4A and S4B). As decreased proliferative28 and migratory29 capacities are well-known senescence-associated phenotypes in multiple cell types of adult stem cells, we investigated whether SHH alleviates senescence-induced stem cell dysfunctions in vitro. As shown in Figures 1D and 1E, SHH markedly alleviated senescence-induced suppressive effects on the growth and migration of endometrial stem cells. To further confirm the alleviating effect of SHH on senescence-induced inhibition of endometrial stem cell migration, western blotting was used to evaluate the expression levels of matrix metalloproteinase 2/9 (MMP-2/9), which play important roles in regulating cell migration (Figure 1F). Additionally, we evaluated the effect of SHH treatment on the in vitro self-renewal capacity of endometrial stem cells. We observed steadily increased proliferation rates in endometrial stem cells treated with SHH compared with the nontreated control cells (Figure S5A). SHH significantly increased also the migratory capacity of endometrial stem cells (Figure S5B). Moreover, SHH treatment significantly enhanced the multilineage differentiation capacity of endometrial stem cells toward osteoblasts in vitro (Figure S5C). Taken together, these results suggest that SHH successfully alleviates various senescence-associated endometrial stem cell dysfunctions in vitro.
SHH-Signaling Integrity Clearly Declines with Aging In Vitro and In Vivo
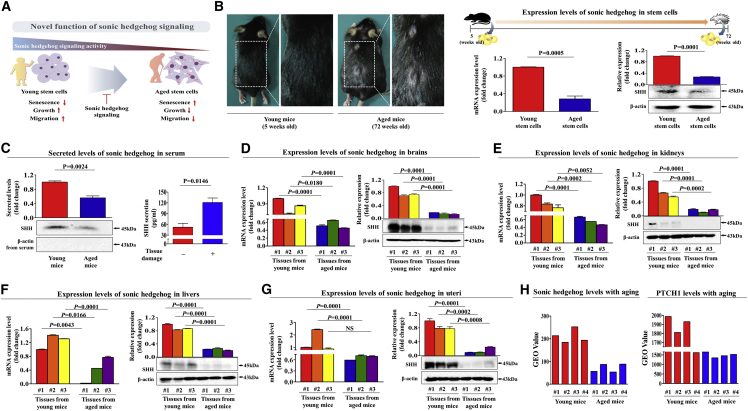
A schematic summary of the main hypothesis of this study is shown in Figure 2A. To investigate whether SHH expression is downregulated in stem cells derived from aged mice in comparison with in stem cells derived from young mice, we performed western blot analysis and real-time PCR. First, we isolated stem cells from mouse adipose tissue (Figure S6A), and their transdifferentiation potential into multiple lineages was evaluated by inducing osteogenic (Figure S6B) and adipogenic (Figure S6C) differentiation. Both SHH mRNA and protein were substantially expressed in adipose tissue-derived stem cells from young mice (5 weeks), but they were only minimally detected in the corresponding cells from aged mice (72 weeks) (Figure 2B). To further verify in vivo whether SHH levels decrease with aging, systemic SHH levels in peripheral blood samples from aged and young mice were examined using both trichloroacetic acid (TCA) precipitation and ELISA (Figure 2C).
Figure 2.
SHH Expression Was Downregulated in Stem Cells with Aging Both In Vitro and In Vivo
Sonic hedgehog (SHH) is considered a morphogen that regulates embryonic development. (A) In addition to this canonical function, SHH may act as an endogenous anti-aging factor. (B) Stem cells from mouse adipose tissue were isolated from young (5-week-old) and aged (72-week-old) mice, and then both mRNA and protein levels of SHH were evaluated using real-time PCR and western blotting, respectively. (C) After albumin and immunoglobulin depletion, the proteins in the serum samples from young and aged mice were precipitated with 10% TCA and subjected to western blotting using an anti-SHH polyclonal antibody. To prove media samples are not contaminated with cytosolic or nuclear content during TCA precipitation procedure, the levels of actin in the culture medium were analyzed. Brain (D), kidney (E), liver (F), and uterus (G) were isolated from young (5-week-old) and aged (72-week-old) mice, and then both mRNA and protein levels of SHH were evaluated using real-time PCR and western blotting, respectively. (H) The GEO database (https://www.ncbi.nlm.nih.gov/geo/) was analyzed to further verify the decreased SHH expression with aging. β-actin was used as an internal control. The results represent the means ± SD from three independent experiments.
To support this observation, we subsequently investigated whether these decreased SHH expression patterns with aging were also consistent with the expression of SHH in other organs, such as the brain, kidney, liver, and uterus. Importantly, both SHH mRNA and protein were significantly decreased in these tissues from aged mice compared with the corresponding tissues from young mice (Figures 2D–2G). Additionally, both SHH mRNA and protein levels of SHH were significantly decreased in endometrial tissues from aged mice compared with the corresponding tissues from young mice (Figure S7A). As expected, SA-β-Gal activity (Figure S7B) and various markers of cellular senescence, such as IL-6, p16, p18, and p21 (Figure S7C), were also significantly increased in endometrial tissues from aged mice. The GEO database was analyzed to further verify the decreased SHH expression with aging. Consistently, the expression levels of SHH and its principal receptor PTCH1 were markedly decreased with aging (Figure 2H). These results suggest that SHH activity clearly declines with aging both in vitro and in vivo as a potent anti-aging factor.
To further confirm whether SHH-signaling integrity declines with aging in a large clinical database, we examined the gene expression profiles using Ingenuity Pathway Analysis (IPA) software. Positive regulators of SHH, such as nuclear factor κB (NF-κB) (Z score = 5.081, p value = 1.86E−01), EGR1 (Z score = 2.868, p value = 2.31E−01), and EZH2 (Z score = 3.065, p value = 3.17E−01), were activated in nonsenescent proliferative cells (Figure S8A). Consistently, positive regulators of PTCH1 (SHH receptor) signaling, such as protein kinase C delta type (PRKCD) (Z score = 4.161, p value = 1.23E−02) and GLI1 (Z score = 3.428, p value = 1.00E−00), were also activated in nonsenescent proliferative cells (Figure S8B).
Additionally, our results from R2: Genomics Analysis and Visualization Platform (https://hgserver1.amc.nl/cgi-bin/r2/main.cgi/), an algorithm for investigating differential gene expression patterns under various pathological conditions, suggest that the expressions of SHH and its receptor PTCH1 significantly decrease as aging progresses in vivo (Figure 3A) and during cellular senescence in vitro (Figure 3B). Moreover, we investigated whether the expression of these genes decreases with aging in vitro and in vivo. We further confirmed that the expression of SHH and its receptor PTCH1 were significantly decreased in both senescent stem cells (Figures S9A and S9B) and various organs of aged mice (Figures 3C and 3D). We also analyzed the activation state of SHH signaling in nonsenescent proliferative cells using GeneMANIA (http://genemania.org/) to evaluate interconnected signaling networks governing growth or migration potential. The results revealed a positive correlation between the activation state of SHH signaling and cell growth or migration (Figure S8C).
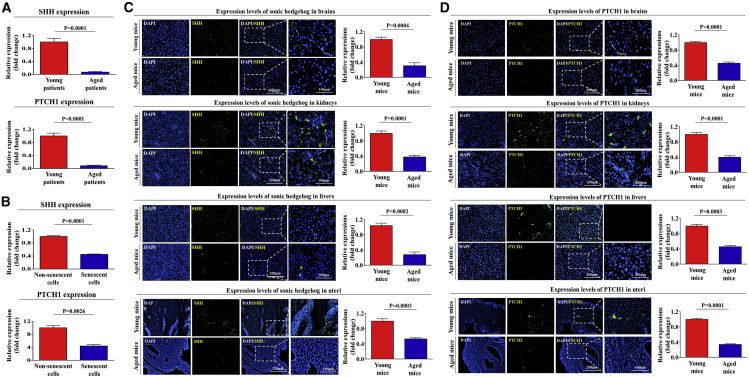
Figure 3.
The Expression Levels of SHH and Its Receptor PTCH1 Were Significantly Decreased with Aging In Vitro and In Vivo
The gene datasets were filtered by the expression profiles of SHH and PTCH1 in young patients (18/24) versus aged patients (21/24) (A) or nonsenescent versus senescent cells (B). Multiple tissues such as brain, kidney, liver, and uterus from young (5-week-old) and aged (72-week-old) mice were stained with antibodies that were specific for SHH (C) and PTCH1 (D). DAPI staining was used to label the nuclei within each field. The results represent the means ± SD from three independent experiments.
SERPINB2 Mediates the Alleviating Effect of SHH on Senescence-Induced Endometrial Stem Cell Dysfunction
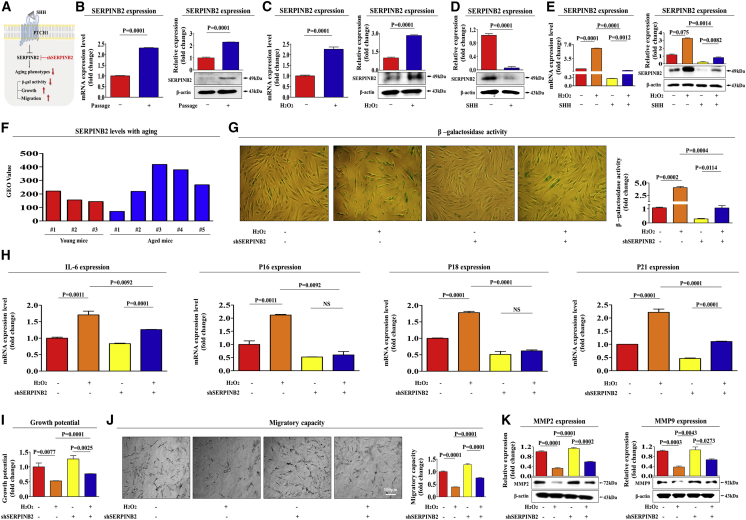
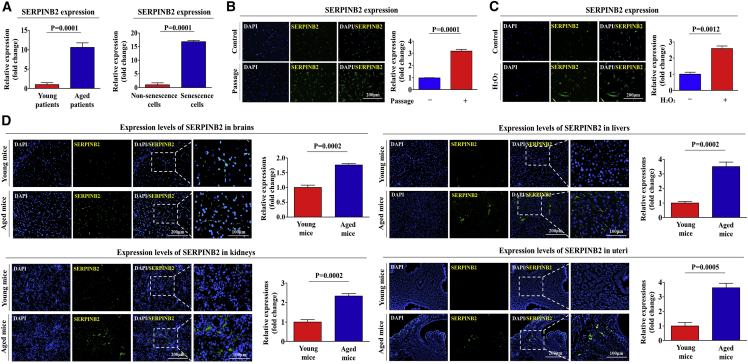
Recent studies have demonstrated that toxic exposure triggers apparent premature senescence in both human and experimental animal models as an age-related risk factor.30, 31 In our previous study, we observed a positive correlation between toxic exposure and significantly enhanced SERPINB2 expression in adult stem cells in vitro and in vivo.32 We therefore hypothesized in the present study that both replicative and oxidative stress-induced cellular senescence may enhance SERPINB2 expression and that it can regulate the effect of SHH on alleviating senescence-induced endometrial stem cell dysfunctions (Figure 4A). Consistent with our hypothesis, replicative (Figure 4B) and oxidative stress-induced (Figure 4C) senescence significantly enhanced SERPINB2 expression at both the mRNA and protein levels. As shown in Figure 4D, SHH treatment markedly suppressed SERPINB2 expression in endometrial stem cells. Importantly, senescence-induced SERPINB2 expression was significantly attenuated by SHH treatment at both the mRNA and protein levels (Figure 4E). Consistently, the GEO database also showed that the expression levels of SERPINB2 were markedly increased with aging (Figure 4F).
Figure 4.
SERPINB2 Mediates the Alleviating Effect of SHH on Endometrial Stem Cell Senescence
(A) Schematic representation describing the functions of SERPINB2 in endometrial stem cells. Replicative (B) and oxidative stress-mediated (C) senescence was induced by continuous subculture until passage 10 and 700 nM hydrogen peroxide (H2O2) exposure for 1 h, respectively. Both mRNA and protein levels of SERPINB2 were assessed by real-time PCR and western blotting, respectively (B and C). (D) Endometrial stem cells were treated with SHH (4 μM), and the protein levels of SERPINB2 were assessed by western blotting. (E) Endometrial stem cells were pretreated with 700 nM hydrogen peroxide (H2O2) for 1 h prior to treatment with 4 μM SHH for 48 h, and the changes in SERPINB2 expression were determined by real-time PCR and western blotting. (F) The GEO database (https://www.ncbi.nlm.nih.gov/geo/) was analyzed to further verify the increased SERPINB2 expression with aging. Endometrial stem cells were transfected with shRNA targeting SERPINB2 with or without oxidative stress-induced senescence (700 nM H2O2); subsequent changes in stem cell aging were determined by measuring SA-β-Gal activity (G) and senescence markers such as IL-6, p16, p18, and p21 (H). (I) The ability of SERPINB2 knockdown to attenuate the senescence-induced inhibitory effects on stem cell viability was measured by an MTT assay. Changes in migratory capacity were measured by the transwell assay (J) and western blotting for MMP-2 and MMP-9 (K). β-actin was used as an internal control. The results represent the means ± SD from three independent experiments.
To further investigate whether SERPINB2 can regulate the effect of SHH on alleviating stem cell senescence, we effectively knocked down its expression using a specific shRNA targeting SERPINB2 (Figures S10A–S10C). Importantly, the effect of SHH on alleviating senescence-induced SA-β-Gal activities (Figure 4G) and the increased expression levels of intracellular or secreted proteins, such as IL-6, p16, p18, and p21 (Figure 4H), were significantly attenuated by SERPINB2 depletion. Consistently, the alleviating effect of SHH on senescence-induced endometrial stem cell dysfunction, such as growth (Figure 4I) and migration (Figures 4J and 4K), was also markedly abolished by SERPINB2 depletion. Taken together, these results suggest that SERPINB2 may be involved in the effect of SHH on alleviating senescence-induced endometrial stem cell dysfunction as a potent downstream regulator of SHH signaling during the aging process.
SERPINB2 Expression Is Significantly Increased with Aging Both In Vitro and In Vivo
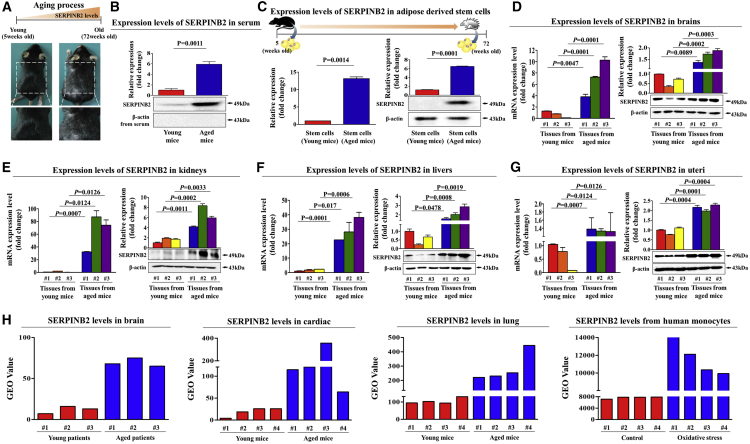
Our in vitro data suggested that SERPINB2 may act as a potent stem cell senescence marker that can mediate the effect of SHH on alleviating stem cell senescence. Therefore, to confirm whether SERPINB2 expression was enhanced in resident stem cells derived from aged mice in comparison with young mice, we performed western blot analysis and real-time PCR. A schematic summary of the main hypothesis is described in Figure 5A. Serum levels of SERPINB2 were significantly increased in aged (72-week-old) mice compared with the corresponding samples from young (5-week-old) mice (Figure 5B). Both mRNA and protein levels of SERPINB2 were substantially expressed in adipose tissue-derived stem cells from aged mice, but they were only minimally detected in the corresponding cells from young mice (Figure 5C).
Figure 5.
SERPINB2 Expression Was Enhanced with Aging Both In Vitro and In Vivo
(A) Schematic representation describing the functions of SERPINB2 with aging. (B) After albumin/immunoglobulin depletion, the proteins in the serum samples from young and aged mice were precipitated with 10% TCA and subjected to western blotting using an anti-SERPINB2 polyclonal antibody. (C) Stem cells from mouse adipose tissue were isolated from young (5-week-old) or aged (72-week-old) mice, and then the protein levels of SERPINB2 were evaluated using real-time PCR and western blotting, respectively. Brain (D), kidney (E), liver (F), and uterus (G) were isolated from young (5-week-old) or aged (72-week-old) mice, and then both mRNA and protein levels of SERPINB2 were evaluated using real-time PCR and western blotting, respectively. (H) The GEO database (https://www.ncbi.nlm.nih.gov/geo/) was analyzed to further verify the increased SERPINB2 expression with aging in multiple organs. β-actin was used as an internal control. The results represent the means ± SD from three independent experiments.
To support this observation, we subsequently investigated whether these increased SERPINB2 expression patterns with aging were also consistent with SERPINB2 expression in other organs, such as the brain, kidney, liver, and uterus. Importantly, both SERPINB2 mRNA and protein were significantly increased in multiple organs from aged mice compared with the corresponding organs from young mice (Figures 5D–5G). Consistently, the GEO database also showed that the expression levels of SERPINB2 were markedly increased in various organs of aged mice (Figure 5H). Moreover, our results from the R2 analysis platform suggested that the expression of SERPINB2 significantly increases as aging or senescence (Figure 6A) progresses. These results suggest that SERPINB2 expression levels clearly increase with aging both in vitro and in vivo as a potent stem cell aging marker. Additionally, we investigated whether SERPINB2 expression increases with age in vitro and in vivo. SERPINB2 expression was significantly increased in both replicative (Figure 6B) and oxidative stress-induced (Figure 6C) senescence. Importantly, SERPINB2 expression was also significantly increased in multiple organs from aged mice compared with the corresponding organs from young mice (Figure 6D).
Figure 6.
Enhanced SERPINB2 Expression Is Associated with the Aging Process in Clinical Big Data
Clinical big data were analyzed using the Seiber dataset (GEO: GSE43996 and GSE9452) from R2: Genomics Analysis and Visualization Platform (https://hgserver1.amc.nl/cgi-bin/r2/main.cgi/). (A) The gene datasets were filtered by the expression profiles of SERPINB2 in young patients (16) versus aged patients (17) or nonsenescent versus senescent cells. Replicative (B) and oxidative stress-mediated (C) senescence was induced by continuous subculture until passage 10 and 700 nM hydrogen peroxide (H2O2) exposure for 1 h, respectively. (D) Cells were stained with antibodies that were specific for SERPINB2. Multiple tissues, such as brain, kidney, liver, and uterus, from young (5-week-old) and aged (72-week-old) mice were stained with antibodies that were specific for SERPINB2. DAPI staining was used to label the nuclei within each field. The results represent the means ± SD from three independent experiments.
To further confirm whether SERPINB2 expression increases with aging, we examined the gene expression profiles of subjects from a large clinical database using IPA software. Positive regulators of SERPINB2, such as SMARCA4 (Z score = −2.536, p value = 4.37E−01) and FOXO1 (Z score = −2.813, p value = 4.28E−02), were also inhibited in nonsenescent proliferative cells (Figure S11A). We also analyzed the activation state of SERPINB2 using GeneMANIA (http://genemania.org/) to evaluate interconnected signaling networks governing growth and migration potential. The results revealed a negative correlation between the activation state of SERPINB2 and cell growth or migration (Figure S11B).
Discussion
The human endometrium is a highly dynamic tissue that undergoes cyclic growth of approximately 7 mm/week during the regular menstrual cycle and develops rich blood vessels for the potential implantation of an embryo.33 This unique regenerative capacity of the endometrium is absolutely required for successful implantation and subsequent reproduction. Stem cells are located in the basal layer of the endometrium, and they are responsible for the cyclic regeneration of the endometrium during the menstrual cycle.34 There is a gradual decline in the regenerative capacities of most tissues with age due to age-associated local stem cell dysfunction.35 Consistently, it has been suggested that aging-related decline in stem cell functions may contribute to various aspects of aging-associated disorders, including ataxia, loss of subcutaneous fat, kyphosis, and sarcopenia.36 Importantly, Lucas et al.8 revealed that consecutive miscarriage is strongly associated with an enhanced endometrial stem cell deficiency and cellular senescence in the uterus. Moreover, the clonogenicity of endometrial stem cells was significantly decreased in 42% of recurrent pregnancy loss (RPL) endometrial biopsies compared with 11% of normal biopsies.8
Due to the close association between local stem cell dysfunction and enhanced endometrial deficiency, increased endometrial stem cell aging may decrease successful implantation and, subsequently, lower pregnancy rates. Consistent with this hypothesis, senescent endometrial stem cells lead to a chronic inflammatory response, which is subsequently associated with susceptibility to RPL.10 Therefore, intensive study of endometrial stem cell aging may provide us with new insights into the mechanisms behind pregnancy failure. Currently, several groups have directed their efforts toward finding novel stimulatory molecules that can prevent or delay the aging process of endometrial stem cells. However, Murakami et al.37 suggested the loss of clonogenic endometrial stem cells is negatively correlated with body mass index (BMI), not aging-associated factors. Cloning efficiency of endometrial stem cell populations was significantly lower in obese subjects compared to subjects with normal BMI. They found no significant results with age. Therefore, further investigation is warranted to explore whether the aging could be considered as a major reason of endometrial stem cell dysfunction.
Most recently, particular attention has been devoted to the noncanonical functions of SHH as a novel endogenous anti-aging factor, as its signaling integrity declines during the development of various aging-associated diseases, such as diabetes,38 neurodegenerative diseases,15 and atherosclerosis.16 Consistent with these observations, Piccioni et al.39 demonstrated that dysregulation of SHH signaling might be a contributing factor to deficient muscle regeneration associated with aging in muscle injury animal models. Matsumoto et al.40 also observed significantly decreased SHH expression in bone marrow cells from the fractured rib site of aged mice compared with the corresponding cells from young mice, which in turn slows bone fracture healing in aged mice. Their results indicated that the age-associated decline in the regenerative potential of multiple tissues may be largely due to the decreased SHH-signaling integrity of local stem cells with aging. However, the direct effects of SHH signaling on various senescence-associated phenotypes of local stem cells and the underlying mechanisms remain unknown.
Consistent with this hypothesis, our results clearly showed that SHH activity declines with aging, both in vitro and in vivo (Figures 1A–1H), and SHH treatment significantly alleviates various aging-associated declines in multiple endometrial stem cell functions (Figures 2A–2F), suggesting that SHH may act as an endogenous anti-aging factor in human endometrial stem cells. Recently, Tersigni et al.41 found higher levels of pro-inflammatory cytokines in endometrial tissues of RPL patients than normal women. These results suggested that the increased levels of circulating pro-inflammatory cytokines with aging are able to induce endometrial inflammation, which in turn may lead to miscarriage. Therefore, further investigation is required to uncover the detailed mechanisms underlying how SHH regulates the inflammatory response in endometrial tissues.
In our previous study, we discovered a significant positive association between toxic exposure and increased SERPINB2 expression in local stem cells in vitro and in vivo.32 We therefore hypothesized that both replicative and oxidative stress-induced senescence may enhance SERPINB2 expression, which in turn mediates the alleviating effect of SHH on senescence-induced endometrial stem cell dysfunctions. Consistently, in the current study, the SERPINB2 mRNA and protein in various aged tissues were significantly increased (Figures 5D–5G), and knockdown of SERPINB2 abrogated the suppressing effects of SHH on the aging-associated declines in multiple endometrial stem cell functions (Figures 4I–4K), suggesting that SERPINB2 is a master regulator of SHH signaling during the aging process. Indeed, other studies revealed that elevated SERPINB2 levels suppress cell growth and are also associated with the enhanced expression of various differentiation-specific markers.42, 43, 44 While SERPINB2 knockdown moderately increased proliferative (Figure 4I) and migratory capacity (Figures 4J and 4K) of endometrial stem cells, the suppression of SERPINB2 expression significantly reduced senescence-induced SA-β-Gal activities (Figure 5H) and various senescence markers (Figure 5I). These results warrant further prospective investigations to verify the reliability of SERPINB2 as a universal and specific biomarker for predicting local stem cell aging, growth, and migration activity.
However, SERPINB2 knockdown using lentiviral shRNA vector in vitro was not sufficient to properly refect the changes of SERPINB2 expression in vivo. The optimal animal model to further confirm the in vivo efficacy of SERPINB2 depletion on various endometrial stem cell functions is SERPINB2-deficient mice. It is, therefore, assumed that performing in vivo experiments with a SERPINB2-deficient mouse model would be sufficient to overcome this major limitation in our future study. Although Eichberger et al.45 previously revealed that the SERPINB2 expression is enhanced in response to GLI (a major downstream target of SHH signaling) during carcinogenesis, the exact molecular mechanisms underlying SHH-mediated SERPINB2 expression in human endometrial stem cells remain unclear. Therefore, further investigation is required to uncover the detailed mechanisms underlying how SHH regulates SERPINB2 expression.
Taken together, this finding suggests that SHH activity clearly declines with aging and subsequently attenuates endometrial stem cell loss and dysfunction in the aging process by suppressing the expression of SERPINB2. This study provides novel insights into the underlying molecular mechanisms regulating the aging of endometrial stem cells with relevance to potential clinical applications. This raises clinically important roles of SHH in protecting the endometrium from age-associated degeneration during in vitro fertilization (IVF) treatment and subsequently increased pregnancy rates. The extent of the observed features contributing to in vitro stem cell aging or dysfunction in the present study, however, may be affected by various concomitant factors, such as donor age, patient pathological conditions, and cell origin, which can yield stem cells with varied characteristics and functionalities and, thus, need to be further investigated.
Materials and Methods
Isolation and Culture of Human Endometrial Stem Cells
Human endometrial stem cells were obtained from endometrial tissues of uterine fibroid patients with written informed consent from the patients and approval of the Gachon University Institutional Review Board (IRB GAIRB2018-134). Endometrial tissue was minced into small pieces, and then the small pieces were digested in DMEM containing 10% fetal bovine serum (FBS) and 250 U/mL type I collagenase for 5 h at 37°C in a rotating shaker. The digestion mixture was then filtered through a 40-μm cell strainer to separate stromal-like stem cells from epithelial gland fragments and undigested tissue. Isolated cells were then cultured in EBM-2 medium (Lonza) with EGM-2 supplements at 37°C and 5% CO2. At least three different human endometrial stem cells were obtained from endometrial tissues and also used for all experiments.
Isolation and Culture of Mouse Adipose Tissue-Derived Stem Cells
The isolation of mouse adipose tissue-derived stem cells was approved and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) (LCDI-2018-0034) of the Lee Gil Ya Cancer and Diabetes Institute of Gachon University. Adipose tissue was minced into small pieces, and then the small pieces were digested in DMEM containing 10% FBS and 250 U/mL type I collagenase for 5 h at 37°C. The digestion mixture was then filtered through a 40-μm cell strainer. Isolated cells were then cultured in EBM-2 medium (Lonza) with EGM-2 supplements at 37°C and 5% CO2.
SA-β-Gal Staining
SA-β-Gal staining was performed as previously described.46 The endometrial stem cells were seeded on 6-well plates at a density of 1 × 105 cells/well. The cells were incubated for 3 days until they reached the appropriate confluency. The cells were then washed twice with PBS and fixed with 0.5% glutaraldehyde in PBS for 5 min. The cells were then washed with PBS containing 1 mM MgCl2 and stained with X-gal solution (1 mg/mL X-gal, 0.12 mM K3Fe(CN)6, and 1 mM MgCl2 in PBS [pH 6.0]) overnight at 37°C.
Cell Proliferation Assay
The 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to determine the anti-proliferative capacity of SHH (Abcam, ab120933), according to the manufacturer’s protocol (Sigma, M5655). Cells (1 × 104 cells/well) were seeded in 96-well plates. After 24 h of incubation, the cells were treated with SHH or vehicle for 72 h. The viable cells were measured at 570 nm using a Versa Max microplate reader.
In Vitro Cell Migration Assay
Cells were plated at 1 × 105 cells/well in 200 μL culture medium in the upper chambers of transwell-permeable supports (Corning, Corning, NY, USA) to track the migration of cells. The transwell chambers had 8.0-μm pores in 6.5-mm diameter polycarbonate membranes and used a 24-well plate format. Non-invading cells on the upper surface of each membrane were removed by scrubbing with laboratory paper. Migrated cells on the lower surface of each membrane were fixed with 4% paraformaldehyde for 5 min and stained with hematoxylin for 15 min. Later, the number of migrated cells was counted in three randomly selected fields of the wells under a light microscope at 50× magnification. To calculate the chemotactic index, the number of cells that migrated in response to the treatment of SHH was divided by the number of spontaneously migrating cells.
Protein Isolation and Western Blot Analysis
The protein expression levels were determined by western blot analysis as previously described.47 Cells were lysed in a buffer containing 50 mM Tris, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP 40, and 0.2 mM PMSF. The protein concentrations of the total cell lysates were measured by using BSA as a standard. Samples containing equal amounts of protein were separated via SDS-PAGE and then transferred onto nitrocellulose membranes (Bio-Rad). The membranes were blocked with 5% skim milk in Tris-buffered saline containing Tween-20 at room temperature (RT). Then the membranes were incubated with primary antibodies against β-actin (Abcam, ab189073), MMP-2 (Cell Signaling Technology, 4022), MMP-9 (Cell Signaling Technology, 13667), SERPINB2 (Abcam, ab47742), SHH (Santa Cruz Biotechnology, SC-373779), or PTCH1 (Abcam, ab53715) overnight at 4°C and then with horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) (BD Pharmingen, 554021) and goat anti-mouse IgG (BD Pharmingen, 554002) secondary antibodies for 60 min at RT. Antibody-bound proteins were detected using enhanced chemiluminescence (ECL) reagents.
Adipogenic Differentiation
Endometrial stem cells were incubated in DMEM low-glucose medium supplemented with 500 μM metylxanthine, 5 μg/mL insulin, and 10% FBS. Endometrial stem cells were grown for 3 weeks, with medium replacement twice a week. Lipid droplet formation was confirmed by oil red O staining. Relative quantification of lipid droplet formation was determined by absorbance measurement at 500 nm.
Osteogenic Differentiation
Endometrial stem cells were incubated in DMEM high-glucose medium supplemented with 0.1 μM dexamethasone, 10 mM β-glycerophosphate, 50 μM ascorbate, and 10% FBS. Endometrial stem cells were grown for 3 weeks, with medium replacement twice a week. Differentiated cells were stained with alizarin red S to detect de novo formation of bone matrix. Alizarin red S in samples was quantified by measuring the optical density (OD) of the solution at 570 nm.
Flow Cytometry
Fluorescence-activated cell sorting (FACS) analysis and cell sorting were performed using FACS Calibur and FACS Aria machines (Becton Dickinson, Palo Alto, CA), respectively. FACS data were analyzed using FlowJo software (Tree Star, Ashland, OR). Antibodies to the following proteins were used: antigen-presenting cell (APC)-conjugated CD44 (BD Biosciences, 559942, dilution 1/40), phycoerythrin (PE)-conjugated CD133 (MACS; Miltenyi Biotech, 130-080-081, dilution 1/40), CD34 (MACS; Miltenyi Biotech, 30-081-002), CD44 (MACS; Miltenyi Biotech, 130-095-180), CD45 (MACS; Miltenyi Biotech, 130-080-201), CD73 (MACS; Miltenyi Biotech, 130-095-182), CD105 (MACS; Miltenyi Biotech, 130-094-941), and CD140b (MACS; Miltenyi Biotech, 130-105-279). The FACS gates were established by staining with an isotype antibody or secondary antibody.
Real-Time PCR
Total RNA from skin cells was extracted using TRIzol reagent (Invitrogen), according to the manufacturer’s protocol. Real-time PCR was performed using a Rotor-Gene Q (QIAGEN). The reaction was subjected to amplification cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 25 s. The relative mRNA expression of the selected genes was normalized to that of peptidylprolyl isomerase A (PPIA) and quantified using the ΔΔCT method. The sequences of the PCR primers are listed in Table 1.
Table 1.
Primer Sequences for qRT-PCR
| Gene | GenBank Accession Number | Direction | Primer Sequence |
|---|---|---|---|
| Human PPIA | NM_021130 | F | 5′-TGCCATCGCCAAGGAGTAG-3′ |
| R | 5′-TGCACAGACGGTCACTCAAA-3′ | ||
| Human SERPINB2 | NM_001143818 | F | 5′-ACCCCCATGACTCCAGAGAACT-3′ |
| R | 5′-GAGAGCGGAAGGATGAATGGAT-3′ | ||
| Human IL-6 | NM_000600 | F | 5′-GGTACATCCTCGACGGCATCT-3′ |
| R | 5′-GTGCCTCTTTGCTGCTTTCAC-3′ | ||
| Human p16 | NM_000077 | F | 5′-CTACTGAGGAGCCAGCGTCT-3′ |
| R | 5′-CTGCCCATCATCATGACCT-3′ | ||
| Human p18 | NM_001262 | F | 5′-TGGGTCTTCCGCAAGAACTC-3′ |
| R | 5′-TGGCAGCCAAGTGCAAGGGC-3′ | ||
| Human p21 | NM_000389 | F | 5′-ACAGCAGAGGAAGACCATGTGGACC-3′ |
| R | 5′-CGTTTTCGACCCTGAGAGTCTCCAG-3′ | ||
| Mouse HPRT | NM_013556 | F | 5′-GCCTAAGATGAGCGCAAGTTG-3′ |
| R | 5′-TACTAGGCAGATGGCCACAGG-3′ | ||
| Mouse SHH | NM_009170 | F | 5′-CTGGCCAGATGTTTTCTGGT-3′ |
| R | 5′-GATGTCGGGGTTGTAATTGG-3′ | ||
| Mouse SERPINB2 | NM_011111 | F | 5′-ACTTAATGGGCTTTATCCTTTCC-3′ |
| R | 5′-TGCGTCCTCAATCTCATCG-3′ |
F, forward; R, reverse.
Immunofluorescent Staining
Samples were fixed with 4% paraformaldehyde for fluorescent staining. Samples were permeabilized with 0.4 M glycine and 0.3% Triton X-100, and nonspecific binding was blocked with 2% normal swine serum (Dako, Glostrup, Denmark). Staining was performed as described previously,48 using the primary anti-SHH (Santa Cruz Biotechnology, SC-373779), PTCH1 (Abcam, ab53715), or SERPINB2 (Abcam, ab47742) antibody. Samples were examined by fluorescence microscopy (Zeiss LSM 510 Meta). The calculation of expression was based on green fluorescence area and density divided by cell number, as determined from the number of DAPI-stained nuclei, in three randomly selected fields for each sample from a total of three independent experiments.
SERPINB2 Knockdown
shRNA targeting SERPINB2 (GenBank: NM_002575) and scrambled shRNA (shCTRL) were purchased from Bioneer (Daejeon, South Korea). For efficient shRNA transfection, reverse transfection was performed using Lipofectamine 2000 (Invitrogen, 52887), according to the manufacturer’s protocol. We chose the SERPINB2 shRNA that is most effective in mRNA levels from five shRNA designed from the target sequence and determined by qRT-PCR analysis.
IPA
An SHH-, PTCH1-, or SERPINB2-related gene analysis was performed with IPA version 2.0 software (Ingenuity Systems, Redwood City, CA). Differentially expressed genes (t test, p < 0.005) between non-senescent cells and senescent cells were subjected to SHH-, PTCH1-, or SERPINB2-related gene analysis (GEO: GSE32474). The significance of each molecule was measured by Fisher’s exact test (p value), which was used to identify differentially expressed genes from the microarray data that overlapped with genes known to be regulated by a molecule. The activation score (Z score) was used to show the status of predicted molecules by comparing the observed differential regulation of genes (up or down) in the microarray data relative to the literature-derived regulation direction, which can be either activating or inhibiting.
R2 Database Analysis
We used the R2: Genomics Analysis and Visualization Platform (https://hgserver1.amc.nl/cgi-bin/r2/main.cgi/) to analyze the expression levels of SHH, PTCH1, and SERPINB2 between young and old patients or non-senescent cells and senescent cells (GEO: GSE32474, THCA cohort dataset, and LAML cohort dataset). The SHH, PTCH1, or SERPINB2 value was log2 transformed and median centered. All of the graphics and statistical values were analyzed by GraphPad Prism 5.0, and p values were calculated by a two-tailed Student’s t test (p < 0.05).
GeneMANIA Algorithm-Based Bioinformatics Analysis
To further analyze genes that interact with or directly regulate SHH, PTCH1, and SERPINB2, we imported all identified genes and their corresponding accession numbers into GeneMANIA (http://genemania.org/). To find gene interactions, we considered several factors, including co-expression, co-localization, and genetic interactions.
Evaluation of SHH and SERPINB2 Levels in Young and Aged Animal Models
All of the animal experiments were approved and conducted in accordance with the IACUC (LCDI-2018-0127) of the Lee Gil Ya Cancer and Diabetes Institute of Gachon University. Mice of each group were sacrificed by cervical dislocation. Brain, kidney, liver, serum, and uterus tissues were obtained from young (5-week-old) and aged (72-week-old) mice. Animal experiments were conducted using 8 mice/group, and 3 representative mice that best reflect the characteristics of each group were selected for data analysis. These tissues were collected and subjected to real-time PCR and western blotting analysis to verify in vivo whether the levels of SHH and SERPINB2 could be differentially regulated with aging.
Statistical Analysis
All the statistical data were analyzed in GraphPad Prism 5.0 (GraphPad, San Diego, CA), and the differences of each group were analyzed using a two-way ANOVA. Values of p < 0.05 were considered to indicate statistical significance.
Author Contributions
A.C., S.-R.P., S.-R.K., S.N., S.L., and C.H.P. conducted the experiments. H.-Y.L. and I.-S.H. designed the experiments and wrote the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1C1B6003442 and NRF-2018R1A2A3074613).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.04.019.
Contributor Information
Hwa-Yong Lee, Email: hylee@jwu.ac.kr.
In-Sun Hong, Email: hongstem@gachon.ac.kr.
Supplemental Information
References
- 1.Lucas E.S., Salker M.S., Brosens J.J. Uterine plasticity and reproductive fitness. Reprod. Biomed. Online. 2013;27:506–514. doi: 10.1016/j.rbmo.2013.06.012. [DOI] [PubMed] [Google Scholar]; Lucas, E.S., Salker, M.S., and Brosens, J.J. (2013). Uterine plasticity and reproductive fitness. Reprod. Biomed. Online 27, 506-514. [DOI] [PubMed]
- 2.Omidvar S., Begum K. Menstrual pattern among unmarried women from south India. J. Nat. Sci. Biol. Med. 2011;2:174–179. doi: 10.4103/0976-9668.92329. [DOI] [PMC free article] [PubMed] [Google Scholar]; Omidvar, S., and Begum, K. (2011). Menstrual pattern among unmarried women from south India. J. Nat. Sci. Biol. Med. 2, 174-179. [DOI] [PMC free article] [PubMed]
- 3.Nelson S.M., Telfer E.E., Anderson R.A. The ageing ovary and uterus: new biological insights. Hum. Reprod. Update. 2013;19:67–83. doi: 10.1093/humupd/dms043. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nelson, S.M., Telfer, E.E., and Anderson, R.A. (2013). The ageing ovary and uterus: new biological insights. Hum. Reprod. Update 19, 67-83. [DOI] [PMC free article] [PubMed]
- 4.de la Fuente R., Bernad A., Garcia-Castro J., Martín M.C., Cigudosa J.C. Retraction: Spontaneous human adult stem cell transformation. Cancer Res. 2010;70:6682. doi: 10.1158/0008-5472.CAN-10-2451. [DOI] [PubMed] [Google Scholar]; de la Fuente, R., Bernad, A., Garcia-Castro, J., Martin, M.C., and Cigudosa, J.C. (2010). Retraction: Spontaneous human adult stem cell transformation. Cancer Res. 70, 6682. [DOI] [PubMed]
- 5.Schultz M.B., Sinclair D.A. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 2016;143:3–14. doi: 10.1242/dev.130633. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schultz, M.B., and Sinclair, D.A. (2016). When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development 143, 3-14. [DOI] [PMC free article] [PubMed]
- 6.Gargett C.E., Nguyen H.P., Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012;13:235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]; Gargett, C.E., Nguyen, H.P., and Ye, L. (2012). Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 13, 235-251. [DOI] [PubMed]
- 7.Gargett C.E., Ye L. Endometrial reconstruction from stem cells. Fertil. Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]; Gargett, C.E., and Ye, L. (2012). Endometrial reconstruction from stem cells. Fertil. Steril. 98, 11-20. [DOI] [PubMed]
- 8.Lucas E.S., Dyer N.P., Murakami K., Lee Y.H., Chan Y.W., Grimaldi G., Muter J., Brighton P.J., Moore J.D., Patel G. Loss of Endometrial Plasticity in Recurrent Pregnancy Loss. Stem Cells. 2016;34:346–356. doi: 10.1002/stem.2222. [DOI] [PubMed] [Google Scholar]; Lucas, E.S., Dyer, N.P., Murakami, K., Lee, Y.H., Chan, Y.W., Grimaldi, G., Muter, J., Brighton, P.J., Moore, J.D., Patel, G., et al. (2016). Loss of Endometrial Plasticity in Recurrent Pregnancy Loss. Stem Cells 34, 346-356. [DOI] [PubMed]
- 9.Goldberg E.L., Dixit V.D. Drivers of age-related inflammation and strategies for healthspan extension. Immunol. Rev. 2015;265:63–74. doi: 10.1111/imr.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]; Goldberg, E.L., and Dixit, V.D. (2015). Drivers of age-related inflammation and strategies for healthspan extension. Immunol. Rev. 265, 63-74. [DOI] [PMC free article] [PubMed]
- 10.Lucas E.S., Dyer N.P., Fishwick K., Ott S., Brosens J.J. Success after failure: the role of endometrial stem cells in recurrent miscarriage. Reproduction. 2016;152:R159–R166. doi: 10.1530/REP-16-0306. [DOI] [PubMed] [Google Scholar]; Lucas, E.S., Dyer, N.P., Fishwick, K., Ott, S., and Brosens, J.J. (2016). Success after failure: the role of endometrial stem cells in recurrent miscarriage. Reproduction 152, R159-R166. [DOI] [PubMed]
- 11.Briscoe J., Thérond P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]; Briscoe, J., and Therond, P.P. (2013). The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416-429. [DOI] [PubMed]
- 12.Young B., Minugh-Purvis N., Shimo T., St-Jacques B., Iwamoto M., Enomoto-Iwamoto M., Koyama E., Pacifici M. Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function. Dev. Biol. 2006;299:272–282. doi: 10.1016/j.ydbio.2006.07.028. [DOI] [PubMed] [Google Scholar]; Young, B., Minugh-Purvis, N., Shimo, T., St-Jacques, B., Iwamoto, M., Enomoto-Iwamoto, M., Koyama, E., and Pacifici, M. (2006). Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function. Dev. Biol. 299, 272-282. [DOI] [PubMed]
- 13.Rimkus T.K., Carpenter R.L., Qasem S., Chan M., Lo H.W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers (Basel) 2016;8:E22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rimkus, T.K., Carpenter, R.L., Qasem, S., Chan, M., and Lo, H.W. (2016). Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers (Basel) 8, E22. [DOI] [PMC free article] [PubMed]
- 14.Rahman M.M., Hazan A., Selway J.L., Herath D.S., Harwood C.A., Pirzado M.S., Atkar R., Kelsell D.P., Linton K.J., Philpott M.P., Neill G.W. A Novel Mechanism for Activation of GLI1 by Nuclear SMO That Escapes Anti-SMO Inhibitors. Cancer Res. 2018;78:2577–2588. doi: 10.1158/0008-5472.CAN-17-2897. [DOI] [PubMed] [Google Scholar]; Rahman, M.M., Hazan, A., Selway, J.L., Herath, D.S., Harwood, C.A., Pirzado, M.S., Atkar, R., Kelsell, D.P., Linton, K.J., Philpott, M.P., and Neill, G.W. (2018). A Novel Mechanism for Activation of GLI1 by Nuclear SMO That Escapes Anti-SMO Inhibitors. Cancer Res. 78, 2577-2588. [DOI] [PubMed]
- 15.Al-Ayadhi L.Y. Relationship between Sonic hedgehog protein, brain-derived neurotrophic factor and oxidative stress in autism spectrum disorders. Neurochem. Res. 2012;37:394–400. doi: 10.1007/s11064-011-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Al-Ayadhi, L.Y. (2012). Relationship between Sonic hedgehog protein, brain-derived neurotrophic factor and oxidative stress in autism spectrum disorders. Neurochem. Res. 37, 394-400. [DOI] [PMC free article] [PubMed]
- 16.Queiroz K.C., Bijlsma M.F., Tio R.A., Zeebregts C.J., Dunaeva M., Ferreira C.V., Fuhler G.M., Kuipers E.J., Alves M.M., Rezaee F. Dichotomy in Hedgehog signaling between human healthy vessel and atherosclerotic plaques. Mol. Med. 2012;18:1122–1127. doi: 10.2119/molmed.2011.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]; Queiroz, K.C., Bijlsma, M.F., Tio, R.A., Zeebregts, C.J., Dunaeva, M., Ferreira, C.V., Fuhler, G.M., Kuipers, E.J., Alves, M.M., Rezaee, F., et al. (2012). Dichotomy in Hedgehog signaling between human healthy vessel and atherosclerotic plaques. Mol. Med. 18, 1122-1127. [DOI] [PMC free article] [PubMed]
- 17.Braungart E., Magdolen V., Degitz K. Retinoic acid upregulates the plasminogen activator system in human epidermal keratinocytes. J. Invest. Dermatol. 2001;116:778–784. doi: 10.1046/j.1523-1747.2001.01310.x. [DOI] [PubMed] [Google Scholar]; Braungart, E., Magdolen, V., and Degitz, K. (2001). Retinoic acid upregulates the plasminogen activator system in human epidermal keratinocytes. J. Invest. Dermatol. 116, 778-784. [DOI] [PubMed]
- 18.Schuster W.A., Medcalf R.L., Kruithof E.K. Retinoic acid potentiates phorbol ester-mediated induction of urokinase and plasminogen activator inhibitor type 2 in human myeloid leukemic cell lines. Endocrinology. 1993;133:1724–1730. doi: 10.1210/endo.133.4.8404615. [DOI] [PubMed] [Google Scholar]; Schuster, W.A., Medcalf, R.L., and Kruithof, E.K. (1993). Retinoic acid potentiates phorbol ester-mediated induction of urokinase and plasminogen activator inhibitor type 2 in human myeloid leukemic cell lines. Endocrinology 133, 1724-1730. [DOI] [PubMed]
- 19.Tapiovaara H., Matikainen S., Hurme M., Vaheri A. Induction of differentiation of promyelocytic NB4 cells by retinoic acid is associated with rapid increase in urokinase activity subsequently downregulated by production of inhibitors. Blood. 1994;83:1883–1891. [PubMed] [Google Scholar]; Tapiovaara, H., Matikainen, S., Hurme, M., and Vaheri, A. (1994). Induction of differentiation of promyelocytic NB4 cells by retinoic acid is associated with rapid increase in urokinase activity subsequently downregulated by production of inhibitors. Blood 83, 1883-1891. [PubMed]
- 20.Montemurro P., Barbuti G., Conese M., Gabriele S., Petio M., Colucci M., Semeraro N. Retinoic acid stimulates plasminogen activator inhibitor 2 production by blood mononuclear cells and inhibits urokinase-induced extracellular proteolysis. Br. J. Haematol. 1999;107:294–299. doi: 10.1046/j.1365-2141.1999.01698.x. [DOI] [PubMed] [Google Scholar]; Montemurro, P., Barbuti, G., Conese, M., Gabriele, S., Petio, M., Colucci, M., and Semeraro, N. (1999). Retinoic acid stimulates plasminogen activator inhibitor 2 production by blood mononuclear cells and inhibits urokinase-induced extracellular proteolysis. Br. J. Haematol. 107, 294-299. [DOI] [PubMed]
- 21.Kovina M.V., Krasheninnikov M.E., Dyuzheva T.G., Danilevsky M.I., Klabukov I.D., Balyasin M.V., Chivilgina O.K., Lyundup A.V. Human endometrial stem cells: High-yield isolation and characterization. Cytotherapy. 2018;20:361–374. doi: 10.1016/j.jcyt.2017.12.012. [DOI] [PubMed] [Google Scholar]; Kovina, M.V., Krasheninnikov, M.E., Dyuzheva, T.G., Danilevsky, M.I., Klabukov, I.D., Balyasin, M.V., Chivilgina, O.K., and Lyundup, A.V. (2018). Human endometrial stem cells: High-yield isolation and characterization. Cytotherapy 20, 361-374. [DOI] [PubMed]
- 22.Toussaint O., Medrano E.E., von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000;35:927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]; Toussaint, O., Medrano, E.E., and von Zglinicki, T. (2000). Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 35, 927-945. [DOI] [PubMed]
- 23.Campisi J. From cells to organisms: can we learn about aging from cells in culture? Exp. Gerontol. 2001;36:607–618. doi: 10.1016/s0531-5565(00)00230-8. [DOI] [PubMed] [Google Scholar]; Campisi, J. (2001). From cells to organisms: can we learn about aging from cells in culture? Exp. Gerontol. 36, 607-618. [DOI] [PubMed]
- 24.Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dimri, G.P., Lee, X., Basile, G., Acosta, M., Scott, G., Roskelley, C., Medrano, E.E., Linskens, M., Rubelj, I., Pereira-Smith, O., et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363-9367. [DOI] [PMC free article] [PubMed]
- 25.Hong I.S., Kang K.S. The effects of Hedgehog on the RNA-binding protein Msi1 in the proliferation and apoptosis of mesenchymal stem cells. PLoS ONE. 2013;8:e56496. doi: 10.1371/journal.pone.0056496. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hong, I.S., and Kang, K.S. (2013). The effects of Hedgehog on the RNA-binding protein Msi1 in the proliferation and apoptosis of mesenchymal stem cells. PLoS ONE 8, e56496. [DOI] [PMC free article] [PubMed]
- 26.Hong I.S., Lee H.Y., Choi S.W., Kim H.S., Yu K.R., Seo Y., Jung J.W., Kang K.S. The effects of hedgehog on RNA binding protein Msi1 during the osteogenic differentiation of human cord blood-derived mesenchymal stem cells. Bone. 2013;56:416–425. doi: 10.1016/j.bone.2013.07.016. [DOI] [PubMed] [Google Scholar]; Hong, I.S., Lee, H.Y., Choi, S.W., Kim, H.S., Yu, K.R., Seo, Y., Jung, J.W., and Kang, K.S. (2013). The effects of hedgehog on RNA binding protein Msi1 during the osteogenic differentiation of human cord blood-derived mesenchymal stem cells. Bone 56, 416-425. [DOI] [PubMed]
- 27.Althubiti M., Lezina L., Carrera S., Jukes-Jones R., Giblett S.M., Antonov A., Barlev N., Saldanha G.S., Pritchard C.A., Cain K., Macip S. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 2014;5:e1528. doi: 10.1038/cddis.2014.489. [DOI] [PMC free article] [PubMed] [Google Scholar]; Althubiti, M., Lezina, L., Carrera, S., Jukes-Jones, R., Giblett, S.M., Antonov, A., Barlev, N., Saldanha, G.S., Pritchard, C.A., Cain, K., and Macip, S. (2014). Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 5, e1528. [DOI] [PMC free article] [PubMed]
- 28.Münz F., Lopez Perez R., Trinh T., Sisombath S., Weber K.J., Wuchter P., Debus J., Saffrich R., Huber P.E., Nicolay N.H. Human mesenchymal stem cells lose their functional properties after paclitaxel treatment. Sci. Rep. 2018;8:312. doi: 10.1038/s41598-017-18862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Munz, F., Lopez Perez, R., Trinh, T., Sisombath, S., Weber, K.J., Wuchter, P., Debus, J., Saffrich, R., Huber, P.E., and Nicolay, N.H. (2018). Human mesenchymal stem cells lose their functional properties after paclitaxel treatment. Sci. Rep. 8, 312. [DOI] [PMC free article] [PubMed]
- 29.Liu M., Lei H., Dong P., Fu X., Yang Z., Yang Y., Ma J., Liu X., Cao Y., Xiao R. Adipose-Derived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties. Cell Transplant. 2017;26:1505–1519. doi: 10.1177/0963689717721221. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, M., Lei, H., Dong, P., Fu, X., Yang, Z., Yang, Y., Ma, J., Liu, X., Cao, Y., and Xiao, R. (2017). Adipose-Derived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties. Cell Transplant. 26, 1505-1519. [DOI] [PMC free article] [PubMed]
- 30.Wan C., Liu J., Nie X., Zhao J., Zhou S., Duan Z., Tang C., Liang L., Xu G. 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS ONE. 2014;9:e89811. doi: 10.1371/journal.pone.0089811. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wan, C., Liu, J., Nie, X., Zhao, J., Zhou, S., Duan, Z., Tang, C., Liang, L., and Xu, G. (2014). 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS ONE 9, e89811. [DOI] [PMC free article] [PubMed]
- 31.Nie X., Liang L., Xi H., Jiang S., Jiang J., Tang C., Liu X., Liu S., Wan C., Zhao J., Yang J. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin induces premature senescence of astrocytes via WNT/β-catenin signaling and ROS production. J. Appl. Toxicol. 2015;35:851–860. doi: 10.1002/jat.3084. [DOI] [PubMed] [Google Scholar]; Nie, X., Liang, L., Xi, H., Jiang, S., Jiang, J., Tang, C., Liu, X., Liu, S., Wan, C., Zhao, J., and Yang, J. (2015). 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin induces premature senescence of astrocytes via WNT/β-catenin signaling and ROS production. J. Appl. Toxicol. 35, 851-860. [DOI] [PubMed]
- 32.Lee N.H., Cho A., Park S.R., Lee J.W., Sung Taek P., Park C.H., Choi Y.H., Lim S., Baek M.K., Kim D.Y. SERPINB2 is a novel indicator of stem cell toxicity. Cell Death Dis. 2018;9:724. doi: 10.1038/s41419-018-0748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, N.H., Cho, A., Park, S.R., Lee, J.W., Sung Taek, P., Park, C.H., Choi, Y.H., Lim, S., Baek, M.K., Kim, D.Y., et al. (2018). SERPINB2 is a novel indicator of stem cell toxicity. Cell Death Dis. 9, 724. [DOI] [PMC free article] [PubMed]
- 33.McLennan C.E., Rydell A.H. Extent of endometrial shedding during normal menstruation. Obstet. Gynecol. 1965;26:605–621. [PubMed] [Google Scholar]; McLennan, C.E., and Rydell, A.H. (1965). Extent of endometrial shedding during normal menstruation. Obstet. Gynecol. 26, 605-621. [PubMed]
- 34.Morelli S.S., Yi P., Goldsmith L.T. Endometrial stem cells and reproduction. Obstet. Gynecol. Int. 2012;2012:851367. doi: 10.1155/2012/851367. [DOI] [PMC free article] [PubMed] [Google Scholar]; Morelli, S.S., Yi, P., and Goldsmith, L.T. (2012). Endometrial stem cells and reproduction. Obstet. Gynecol. Int. 2012, 851367. [DOI] [PMC free article] [PubMed]
- 35.Marędziak M., Marycz K., Tomaszewski K.A., Kornicka K., Henry B.M. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:2152435. doi: 10.1155/2016/2152435. [DOI] [PMC free article] [PubMed] [Google Scholar]; Marędziak, M., Marycz, K., Tomaszewski, K.A., Kornicka, K., and Henry, B.M. (2016). The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2152435. [DOI] [PMC free article] [PubMed]
- 36.George S.K., Jiao Y., Bishop C.E., Lu B. Mitochondrial peptidase IMMP2L mutation causes early onset of age-associated disorders and impairs adult stem cell self-renewal. Aging Cell. 2011;10:584–594. doi: 10.1111/j.1474-9726.2011.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; George, S.K., Jiao, Y., Bishop, C.E., and Lu, B. (2011). Mitochondrial peptidase IMMP2L mutation causes early onset of age-associated disorders and impairs adult stem cell self-renewal. Aging Cell 10, 584-594. [DOI] [PMC free article] [PubMed]
- 37.Murakami K., Bhandari H., Lucas E.S., Takeda S., Gargett C.E., Quenby S., Brosens J.J., Tan B.K. Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure--a pilot study. PLoS ONE. 2013;8:e82582. doi: 10.1371/journal.pone.0082582. [DOI] [PMC free article] [PubMed] [Google Scholar]; Murakami, K., Bhandari, H., Lucas, E.S., Takeda, S., Gargett, C.E., Quenby, S., Brosens, J.J., and Tan, B.K. (2013). Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure--a pilot study. PLoS ONE 8, e82582. [DOI] [PMC free article] [PubMed]
- 38.Thomas M.K., Rastalsky N., Lee J.H., Habener J.F. Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes. 2000;49:2039–2047. doi: 10.2337/diabetes.49.12.2039. [DOI] [PubMed] [Google Scholar]; Thomas, M.K., Rastalsky, N., Lee, J.H., and Habener, J.F. (2000). Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes 49, 2039-2047. [DOI] [PubMed]
- 39.Piccioni A., Gaetani E., Neri V., Gatto I., Palladino M., Silver M., Smith R.C., Giarretta I., Pola E., Hlatky L., Pola R. Sonic hedgehog therapy in a mouse model of age-associated impairment of skeletal muscle regeneration. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:245–252. doi: 10.1093/gerona/glt076. [DOI] [PMC free article] [PubMed] [Google Scholar]; Piccioni, A., Gaetani, E., Neri, V., Gatto, I., Palladino, M., Silver, M., Smith, R.C., Giarretta, I., Pola, E., Hlatky, L., and Pola, R. (2014). Sonic hedgehog therapy in a mouse model of age-associated impairment of skeletal muscle regeneration. J. Gerontol. A Biol. Sci. Med. Sci. 69, 245-252. [DOI] [PMC free article] [PubMed]
- 40.Matsumoto K., Shimo T., Kurio N., Okui T., Obata K., Masui M., Pang P., Horikiri Y., Sasaki A. Expression and Role of Sonic Hedgehog in the Process of Fracture Healing with Aging. In Vivo. 2016;30:99–105. [PubMed] [Google Scholar]; Matsumoto, K., Shimo, T., Kurio, N., Okui, T., Obata, K., Masui, M., Pang, P., Horikiri, Y., and Sasaki, A. (2016). Expression and Role of Sonic Hedgehog in the Process of Fracture Healing with Aging. In Vivo 30, 99-105. [PubMed]
- 41.Tersigni C., D’Ippolito S., Di Nicuolo F., Marana R., Valenza V., Masciullo V., Scaldaferri F., Malatacca F., de Waure C., Gasbarrini A. Recurrent pregnancy loss is associated to leaky gut: a novel pathogenic model of endometrium inflammation? J. Transl. Med. 2018;16:102. doi: 10.1186/s12967-018-1482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tersigni, C., D’Ippolito, S., Di Nicuolo, F., Marana, R., Valenza, V., Masciullo, V., Scaldaferri, F., Malatacca, F., de Waure, C., Gasbarrini, A., et al. (2018). Recurrent pregnancy loss is associated to leaky gut: a novel pathogenic model of endometrium inflammation? J. Transl. Med. 16, 102. [DOI] [PMC free article] [PubMed]
- 42.Jang S., Yang T.H., An E.J., Yoon H.K., Sohn K.C., Cho A.Y., Ryu E.K., Park Y.S., Yoon T.Y., Lee J.H., Kim C.D. Role of plasminogen activator inhibitor-2 (PAI-2) in keratinocyte differentiation. J. Dermatol. Sci. 2010;59:25–30. doi: 10.1016/j.jdermsci.2010.04.012. [DOI] [PubMed] [Google Scholar]; Jang, S., Yang, T.H., An, E.J., Yoon, H.K., Sohn, K.C., Cho, A.Y., Ryu, E.K., Park, Y.S., Yoon, T.Y., Lee, J.H., and Kim, C.D. (2010). Role of plasminogen activator inhibitor-2 (PAI-2) in keratinocyte differentiation. J. Dermatol. Sci. 59, 25-30. [DOI] [PubMed]
- 43.Jensen P.J., Wu Q., Janowitz P., Ando Y., Schechter N.M. Plasminogen activator inhibitor type 2: an intracellular keratinocyte differentiation product that is incorporated into the cornified envelope. Exp. Cell Res. 1995;217:65–71. doi: 10.1006/excr.1995.1064. [DOI] [PubMed] [Google Scholar]; Jensen, P.J., Wu, Q., Janowitz, P., Ando, Y., and Schechter, N.M. (1995). Plasminogen activator inhibitor type 2: an intracellular keratinocyte differentiation product that is incorporated into the cornified envelope. Exp. Cell Res. 217, 65-71. [DOI] [PubMed]
- 44.Robinson N.A., Lapic S., Welter J.F., Eckert R.L. S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J. Biol. Chem. 1997;272:12035–12046. doi: 10.1074/jbc.272.18.12035. [DOI] [PubMed] [Google Scholar]; Robinson, N.A., Lapic, S., Welter, J.F., and Eckert, R.L. (1997). S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J. Biol. Chem. 272, 12035-12046. [DOI] [PubMed]
- 45.Eichberger T., Sander V., Schnidar H., Regl G., Kasper M., Schmid C., Plamberger S., Kaser A., Aberger F., Frischauf A.M. Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics. 2006;87:616–632. doi: 10.1016/j.ygeno.2005.12.003. [DOI] [PubMed] [Google Scholar]; Eichberger, T., Sander, V., Schnidar, H., Regl, G., Kasper, M., Schmid, C., Plamberger, S., Kaser, A., Aberger, F., and Frischauf, A.M. (2006). Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics 87, 616-632. [DOI] [PubMed]
- 46.Yu K.R., Lee J.Y., Kim H.S., Hong I.S., Choi S.W., Seo Y., Kang I., Kim J.J., Lee B.C., Lee S. A p38 MAPK-mediated alteration of COX-2/PGE2 regulates immunomodulatory properties in human mesenchymal stem cell aging. PLoS ONE. 2014;9:e102426. doi: 10.1371/journal.pone.0102426. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yu, K.R., Lee, J.Y., Kim, H.S., Hong, I.S., Choi, S.W., Seo, Y., Kang, I., Kim, J.J., Lee, B.C., Lee, S., et al. (2014). A p38 MAPK-mediated alteration of COX-2/PGE2 regulates immunomodulatory properties in human mesenchymal stem cell aging. PLoS ONE 9, e102426. [DOI] [PMC free article] [PubMed]
- 47.Choi E.S., Jung J.Y., Lee J.S., Park J.H., Cho N.P., Cho S.D. Myeloid cell leukemia-1 is a key molecular target for mithramycin A-induced apoptosis in androgen-independent prostate cancer cells and a tumor xenograft animal model. Cancer Lett. 2013;328:65–72. doi: 10.1016/j.canlet.2012.09.009. [DOI] [PubMed] [Google Scholar]; Choi, E.S., Jung, J.Y., Lee, J.S., Park, J.H., Cho, N.P., and Cho, S.D. (2013). Myeloid cell leukemia-1 is a key molecular target for mithramycin A-induced apoptosis in androgen-independent prostate cancer cells and a tumor xenograft animal model. Cancer Lett. 328, 65-72. [DOI] [PubMed]
- 48.Dong H.J., Jang G.B., Lee H.Y., Park S.R., Kim J.Y., Nam J.S., Hong I.S. The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells. Sci. Rep. 2016;6:22966. doi: 10.1038/srep22966. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dong, H.J., Jang, G.B., Lee, H.Y., Park, S.R., Kim, J.Y., Nam, J.S., and Hong, I.S. (2016). The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells. Sci. Rep. 6, 22966. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.