Abstract
Background
Recurrent implantation failure is a critical issue in IVF‐ET treatment. Successful embryo implantation needs appropriate molecular and cellular communications between embryo and uterus. Rodent models have been used intensively to understand these mechanisms.
Methods
The molecular and cellular mechanisms of embryo implantation were described by referring to the previous literature investigated by us and others. The studies using mouse models of embryo implantation were mainly cited.
Results
Progesterone (P4) produced by ovarian corpus luteum provides the uterus with receptivity to the embryo, and uterine epithelial growth arrest and stromal proliferation, what we call uterine proliferation‐differentiation switching (PDS), take place in the peri‐implantation period before embryo attachment. Uterine PDS is a hallmark of uterine receptivity, and several genes such as HAND2 and BMI1, control uterine PDS by modulating P4‐PR signaling. As the next implantation process, embryo attachment onto the luminal epithelium occurs. This process is regulated by FOXA2‐LIF pathway and planar cell polarity signaling. Then, the luminal epithelium at the embryo attachment site detaches from the stroma, which enables trophoblast invasion. This process of embryo invasion is regulated by HIF2α in the stroma.
Conclusion
These findings indicate that embryo implantation contains multistep processes regulated by specific molecular pathways.
Keywords: cell proliferation, embryo implantation, infertility, mouse models, uterine receptivity
1. INTRODUCTION
Infertility is a global issue to influence ~10% of reproductive‐age couples.1 However in some developing countries, infertility rates are much higher, reaching 30%.1, 2, 3, 4 Although there are many causes of infertility, infertile patients eventually undergo IVF‐ET. Some of the infertile patients suffer recurrent implantation failure, which is a serious issue in infertility treatment. Embryo implantation is a series of molecular interactions between the embryo and the maternal uterus. It consists of the following three steps: embryo apposition, attachment, and invasion. An embryo attaches to the receptive uterine epithelium, and then, invades into the uterine stroma underneath the luminal epithelium. Successful implantation is the result of appropriate molecular communications between the embryo and uterus during these steps.5, 6, 7, 8 Since a previous study showed that implantation failure causes 75% of failed conceptions,9 it is necessary to elucidate the mechanism of implantation failure for the purpose of increasing the rate of pregnancy and live birth.
In implantation studies, researchers have often used animals, especially mice.5, 8 Recent genetically engineered mouse models rendered valuable information about the detailed mechanisms in embryo implantation.10, 11, 12 This article introduces the evidence of embryo implantation to help better understanding molecular mechanisms of embryo implantation.
2. HORMONAL CONTROL OF EMBRYO IMPLANTATION IN MICE
Progesterone (P4) plays a key role in each step of pregnancy.10, 11, 12, 13 After ovulation, ovarian corpus luteum secretes P4. Luteolysis is inhibited by successful implantation, and corpus luteum keeps secreting P4. In mice, vaginal plug is seen in the morning on the next day of mating and ovulation, and this day is defined as day 1 of pregnancy. The luminal epithelium proliferates prominently, and the uterus looks swollen under the influence of 17β‐estradiol (E2) surge. Serum P4 level is increased on day 3 of pregnancy because newly formed corpus luteum starts to produce P4 markedly after ovulation. By day 4 morning, P4 overcomes E2 as a dominant hormone and heightened P4 provides uterine receptivity to the embryo. The luminal epithelium declines to proliferate and concurrently differentiates; on the other hands, stroma starts to proliferate,14 and this event is called as uterine proliferation‐differentiation switching (PDS). A minor E2 surge with high circulating levels of P4 on late day 4 morning initiates embryo‐uterine communications on day 4 evening. Dormant blastocysts are activated by E2, and the uterus becomes receptive. Therefore, both receptive uterus and competent blastocysts are required for the molecular and cellular communications with each other under the influence of ovarian steroids.5, 8 Then, an intimate adherence of the trophectoderm to the luminal epithelium takes place on day 4 midnight. Stromal cells neighboring the blastocyst start differentiation, change their morphology into the epithelioid shape, and produce a new layer surrounding the blastocyst. This is the process of decidualization. The attachment reaction is accompanied by the increase in stromal vascular permeability at the site of the blastocyst, where can be visualized by Chicago blue dye solution which is injected intravenously. On day 5 evening, trophoblast cells enter the stromal layer of the endometrium. Thus, embryo implantation is completed.5, 8, 15
3. UTERINE PDS AND RECEPTIVITY IN MICE
The following two components are essential for successful embryo implantation: a competent blastocyst and uterine receptivity. The latter is defined as a capacity to accept the competent blastocyst in the uterus.5, 8 Low‐quality embryo causes implantation failure.16 The uterus with receptivity to the embryo shows a suitable uterine preparation with epithelial differentiation and stromal proliferation called as PDS, which is stimulated by ovarian steroids and a hallmark of uterine receptivity (Figure 1).14 In this process, P4 changes the stromal morphology and this phenomenon is called “pre‐decidualization”.7 Then, a spike of ovarian E2 with increased P4 converts the uterus into the receptive state. It is presumed that endometrium‐derived factors endow dormant blastocysts with the competency for blastocyst attachment.5 Once the blastocyst attaches to the endometrium, the receptive uterus enters the refractory state in which any competent blastocysts cannot adhere to the endometrium. This limited duration of uterine capacity for blastocyst attachment is called as “implantation window”.7 The luminal epithelium at the lateral side of the embryo attachment site detaches itself from the stroma and then trophoblast starts to invade the stroma, which is called “embryo invasion”.7, 15 Thus, successful implantation is controlled by uterine receptivity precisely, and uterine PDS is a major indicator of uterine receptivity.
Figure 1.
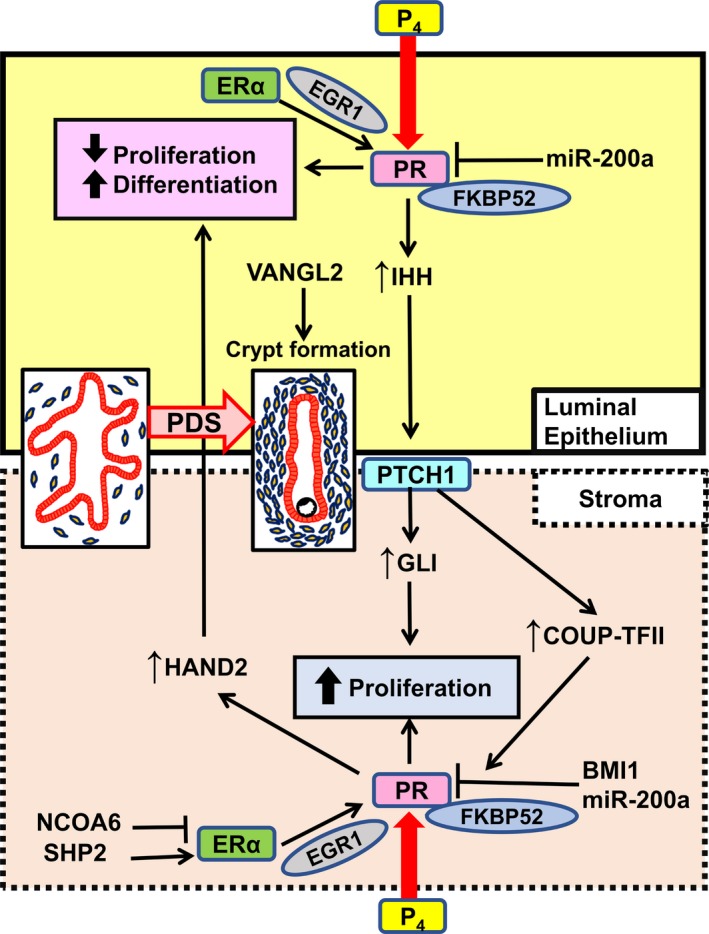
Molecular pathways involved in uterine proliferation‐differentiation switching (PDS). Progesterone, P4; progesterone receptor, PR; 52‐kDa FK506 binding protein, FKBP52; microRNA‐200a, miR‐200a; Indian hedgehog, IHH; Van Gogh‐like 2, VANGL2; patched‐1, PTCH1; COUP transcription factor 2, COUP‐TFII; B lymphoma Mo‐MLV insertion region 1 homolog, BMI1; nuclear receptor co‐activator 6, NCOA6; SRC homology 2 domain‐containing protein tyrosine phosphatase‐2, SHP2; estrogen receptor α, ERα; early growth response protein 1, EGR1; heart and neural crest derivatives‐expressed protein 2, HAND2
4. P4‐PR SIGNALING IN EMBRYO IMPLANTATION
In the clinical setting, progestin including P4 improves implantation rate by supporting the function of corpus luteum; therefore, details of P4 action should be clarified to develop new approaches to the infertility treatment.17
P4 acts through P4 receptor (PR), a nuclear receptor, transcriptionally controlling the P4 responsive genes and the important pathways for pregnancy events, such as ovulation and implantation.5, 18 Studies using the mouse models targeting PR and its related molecules gradually revealed P4 roles in pregnancy. PR null female mice are infertile due to ovulation failure,10 indicating that P4‐PR signaling is crucial for ovulation. Thus, PR knockout mouse is a useful model to analyze the molecular pathways in ovulation. However, this model cannot clarify the effects of P4 on embryo implantation.
PR function is influenced by the stability of PR complex. Functionally, mature PR complex consists of a receptor monomer, a 90‐kDa heat shock protein (Hsp90) dimer, a cochaperone p23, and one of four cochaperones which include tetratricopeptide repeat (TPR) that binds to Hsp90.19 The immunophilin cochaperone 52‐kDa FK506 binding protein (FKBP52) is one of such TPR‐containing cochaperones, binding both Hsp90 and PR, stabilizing the structure of PR complex, and enhancing P4‐PR signaling.12, 19, 20 FKBP52 null mice are infertile specifically due to defected implantation resulting from the impairment of uterine receptivity. Deficiency of FKBP52 diminishes uterine P4‐PR signaling. It does not break up the signal completely, because minimal binding of P4 to PR is still alive.12, 19, 20 Excessive P4 administration can rescue uterine PR signaling in FKBP52 deficient mice on the CD1 background. This is not a remarkable aspect of PR knockout mice, but that of FKBP52 null mice.12 Moreover, FKBP52 null mice show normal ovulation with normal P4 secretion.12 Therefore, FKBP52 deficient mouse is a well‐established unique model with uterine “P4 resistance,” which means that P4 responsiveness is diminished, but is reversible with P4 administration. Taken together, P4‐PR signaling is a crucial pathway for embryo implantation.
5. P4‐PR SIGNALING CONTROLS UTERINE PDS AND RECEPTIVITY
Uterine PDS in the receptive uterus is observed in humans as well as in mice.14 Generally speaking, cell differentiation and poor cell proliferation can be observed simultaneously, and distinct switching between proliferation and differentiation occurs in many cell types.21, 22, 23, 24 Our previous study showed that FKBP52 null mice have continuous epithelial proliferation without enhanced stromal proliferation on day 4 morning, and these phenotypes are recovered by P4 supplementation, indicating uterine P4 resistance in FKBP52 knockout mice. PR antagonist RU486 injection in the peri‐implantation period hampers uterine PDS and embryo implantation in wild‐type (WT) mice.14 According to the previous literature, implantation failure occurs in all types of mice with impaired uterine PDS.14, 15, 19, 25, 26, 27, 28, 29 PR has two isoforms, PR‐A and PR‐B. Previous studies demonstrated that PR‐A is mainly associated with uterine function during pregnancy, contributing to uterine PDS.11, 30 In contrast, PR‐B null mice have normal pregnancy outcome, which is presumed that PR‐B does is not important for pregnancy process. These findings suggest that P4‐PR‐A signaling governs uterine receptivity by controlling uterine PDS (Figures 1 and 2).
Figure 2.
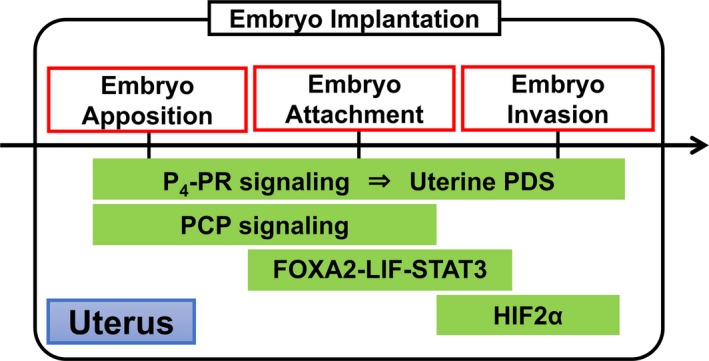
Key signals and pathways in the multistep processes of embryo implantation. Progesterone, P4; progesterone receptor, PR; proliferation‐differentiation switching, PDS; planar cell polarity, PCP; forkhead box protein A2, FOXA2; leukemia inhibitory factor, LIF; signal transducer and activator of transcription 3, STAT3; hypoxia‐inducible factor 2α, HIF2α
6. APPROPRIATE BALANCE BETWEEN E2 AND P4 IS NECESSARY FOR UTERINE PDS AND RECEPTIVITY
The regulation of appropriate balance between E2 and P4 is a delicate mechanism to induce uterine PDS. In mice, a spike of E2 secretion from ovary just before implantation strictly controls the “implantation window.” Neither lack nor excess of E2 level can open the implantation window.31 In the condition of excess E2‐estrogen receptor (ER) signaling in humans, implantation failure occurs at higher rates.32, 33, 34, 35 Abnormal balance between E2‐ER signaling and P4‐PR signaling leads to implantation failure in the mouse models other than FKBP52 deficient mice. In mice with uterine deficiency of nuclear receptor co‐activator 2 (NCOA2), gene encoding steroid receptor co‐activator 2 (SRC2), the disrupt of the optimization of PR function by NCOA2 causes implantation failure.36 Although previous in vitro studies demonstrated that nuclear receptor co‐activator 6 (NCOA6) interacts with ERα as a co‐activator,37, 38, 39, 40 an in vivo study reported that NCOA6 does not work as co‐activator but induces the ubiquitination and degradation of ERα, diminishing E2‐ER signaling in the peri‐implantation period27 (Figure 1). By uterine deletion of NCOA6, ERα is accumulated and E2 sensitivity is enhanced, resulting in aberrant E2/P4 signaling balance and implantation failure.27 Intriguingly, the treatment with ER antagonist ICI‐182780 can rescue not only this hormonal signaling imbalance but also implantation failure.27 Protein tyrosine phosphatase SHP2, classic cytoplasmic protein, is present mainly in the nucleus of endometrial cells during implantation, and nuclear SHP2 enhances SRC kinase‐mediated ERα tyrosine phosphorylation, assists combining ERα with PR promoter, and proceeds the ERα transcription activity in the peri‐implantation period28 (Figure 1).
Uterine‐specific deletion of signal transducer and activator of transcription 3 (STAT3), known as a downstream molecule of leukemia inhibitory factor (LIF) before implantation,41 also induces implantation failure due to the effect of E2‐ER signaling rather than that of P4‐PR signaling in the peri‐implantation period,42 but the minute interaction between STAT3 and E2/P4 signaling is not fully revealed.
A recent study of mouse models demonstrated that uterine ablation of the polycomb group gene BMI1, a component of the polycomb repressive complex‐1 (PRC1), induces implantation failure due to uterine P4 responsiveness.29 BMI1 interacts with PR and E3 ligase E6AP in a polycomb complex‐independent manner and controls PR ubiquitination.29 In women who had a spontaneous miscarriage, low BMI1 expression in endometrium is correlated with poor PR responsiveness.29 Thus, BMI1 controls uterine PR function under the post‐transcriptional modification and contributes to successful embryo implantation in mice and humans (Figure 1).
7. P4‐INDUCED UTERINE PDS IS MODIFIED BY HAND2, IHH, AND EGR1
Heart and neural crest derivatives‐expressed protein 2 (HAND2), one of basic helix‐loop‐helix transcription factors, in the uterine stroma, influences P4‐PR signaling and hampers epithelial proliferation by blocking the stromal expression of fibroblast growth factor, whereas it does not have effect on stromal proliferation.26 HAND2 has a role of a blocker of epithelial E2 signaling, permitting the preparation of the uterine epithelium for embryo implantation. Uterine deletion of HAND2 leads to impairment of embryo attachment, suggesting that HAND2 in the stroma regulates embryo attachment through the process of epithelial differentiation induced by P4 (Figure 1).
Indian hedgehog (IHH) is a downstream factor of PR and is highly expressed in the uterine luminal epithelium of WT mice just before embryo attachment, and also in the endometrium of humans with the progestin treatment.43, 44, 45 IHH works through its receptor patched‐1 (PTCH1), localized in the uterine stroma, and prompts to stromal proliferation.43, 44, 45 The downstream targets of IHH pathway are GLI, which is one of transcriptional factors, and a nuclear receptor chicken ovalbumin upstream promoter‐transcriptional factor (COUP‐TFII).43, 46 GLI may contribute to stromal proliferation,43 and COUP‐TFII may keep the appropriate balance between the ER and PR signaling46 (Figure 1). These findings suggest the presence of complicated but regulated interaction between uterine epithelium and stroma under hormonal control.
Another recent study showed that early growth response 1 (EGR1) null female mice are completely infertile due to implantation failure.47 EGR1 belongs to the EGR family of zinc finger transcription factors which participate in the regulation of cell proliferation, differentiation, and apoptosis.48, 49 EGR1 is induced in both epithelial cells and stromal cells by E2 through the ERα‐ERK1/2 pathway in the uterus50 (Figure 1) and also induced in the subluminal stromal cells surrounding the implanting blastocyst.50, 51 In EGR1 null mice, the expression of PR in epithelial cells is aberrantly reduced, E2 activity is enhanced, and P4 response is impaired.47 Furthermore, the uterus of EGR1 null mice demonstrated continuous proliferation of luminal epithelial cells and poor proliferation of stromal cells,47 indicating that impaired uterine PDS in EGR1 null mice. These findings suggest that E2 induces EGR1 to fine‐tune its actions on uterine epithelium by controlling P4‐PR signaling in order to acquire uterine receptivity.47
8. UTERINE MICRORNA REGULATES P4‐PR SIGNALING AND PDS EPIGENETICALLY
We previously demonstrated that PDS takes place in a spatial manner, between the uterine corpus and cervix.14 The place where blastocyst implantation occurs under the normal pregnancy is the endometrium in the uterine corpus, but not the uterine cervix. In the peri‐implantation period, PDS is recognized in the mouse uterine corpus, but not in the uterine cervix. The human endometrium in the uterine corpus also exhibits dynamic PDS from the proliferative phase to the secretory phase, while the human uterine cervix does not show any significant changes of the proliferation status.14 Based on these findings, we speculated the presence of distinct regulation system of P4‐PR signaling between the uterine corpus and cervix. Interestingly, we found that P4‐PR signaling is down‐regulated in the uterine cervix by microRNA (miR)‐200a in two separate pathways. First, decrease in miR‐200a reduces the expression levels of PR protein by post‐transcriptional regulation.14 Second, miR‐200a up‐regulates 20α‐hydroxysteroid dehydrogenase (20α‐HSD), a P4‐metabolizing enzyme, through down‐regulation of STAT5, consistent with previous reports,52 indicating that miR‐200a reduces local concentration of P4 in the uterine cervix. Moreover, we demonstrated that miR‐200a expression is down‐regulated at the receptive endometrium in the uterine corpus rather than the pre‐receptive one, suggesting that miR‐200a contributes to successful implantation through the regulation of uterine P4‐PR signaling (Figure 1).
9. EMBRYO ATTACHMENT IS REGULATED BY UTERINE FOXA2‐LIF PATHWAY AND PLANAR CELL POLARITY (PCP) SIGNALING
Forkhead Box A2 (FOXA2) controls embryo attachment,53 and Vang‐like protein 2 (VANGL2) induces crypt formation of implantation sites and appropriate embryo attachment.54 As described above, E2 is an initiator for embryo attachment. Leukemia inhibitory factor (LIF), an interleukin‐6 (IL‐6) family cytokine, is produced by endometrial glands in response to E2 secreted by ovaries and has a very important role in embryo attachment. In a delayed implantation mouse model, which is ovariectomized on day 4 of pregnancy and received hormone supplementation later, LIF causes embryo attachment instead of E2.55 A recent study revealed that FOXA2 is expressed in the uterine glandular epithelium and essential for uterine glands development in neonatal mice.53 FOXA2 deletion in the entire uterus and in the epithelium causes complete loss of uterine gland, and embryo attachment failure due to LIF reduction, respectively.53 Attachment failure in the latter mice is recovered by LIF supplementation.53 Taken together, E2‐FOXA2‐LIF pathway has a critical role in embryo attachment (Figure 2).
In mice, embryo attachment occurs at the bottom of crypts, which originate as epithelial evaginations from the main lumen at orderly spaced intervals.54 However, the mechanism of epithelial evaginations was not clarified. Planar cell polarity (PCP) is known as a controller which directs actin‐dependent morphogenetic cell movement to polarize structures in a wide range of settings.56 A recent study showed that VANGL2, which is a core PCP component and works to execute PCP signaling in collaboration with many other molecules, has a crucial role in uterine crypt formation and embryo attachment54 (Figure 1). The litter size is significantly reduced in mice with uterine VANGL2 deletion. Uterine deletion of VANGL2 confers aberrant PCP signaling, misdirected epithelial evaginations, defective crypt formation, and embryo attachment, leading to severely compromised pregnancy outcomes.54 These findings suggest that PCP signaling is crucial for embryo implantation (Figure 2).
10. EMBRYO INVASION IS REGULATED BY HIF2α IN THE STROMA
The mechanisms of embryo invasion have not been elucidated. Since the surface of the endometrium is far from uterine blood vessels, it is possible that oxygen concentration in the luminal epithelium is relatively low compared with the inner endometrium.57 Therefore, it is speculated that the surface of endometrium is in hypoxic state during embryo implantation. Hypoxia‐inducible factor (HIF) is a common transcriptional factor induced by low oxygen tension.58 In mice, uterine HIF2α expression is intense during peri‐implantation period.59 We recently revealed that entire uterine deletion of HIF2α results in implantation failure due to embryo invasion failure in mice15 (Figure 2). Supplementation of both P4 and LIF does not rescue embryo invasion but recovers decidual growth arrest and inappropriate location of implantation site in uterine HIF2α knockout mice. Notably, embryo invasion failure in uterine HIF2α null mice is caused by the intact alignment of luminal epithelium, which hampers direct attachment of embryo to uterine stroma, and inactivation of AKT pathway as an embryonic survival signal.15 Uterine stromal HIF2α knockout mice are infertile due to impaired embryo invasion, whereas uterine epithelial HIF2α knockout mice demonstrate normal fertility, indicating the critical role of uterine stromal HIF2α in embryo invasion. This study offers new insight that stromal HIF2α controls trophoblast invasion into the endometrium through detachment of luminal epithelium and activation of an embryonic survival signal (Figure 3). Ultimately, we could discover HIF2α as a novel factor controlling embryo invasion (Figure 2).
Figure 3.
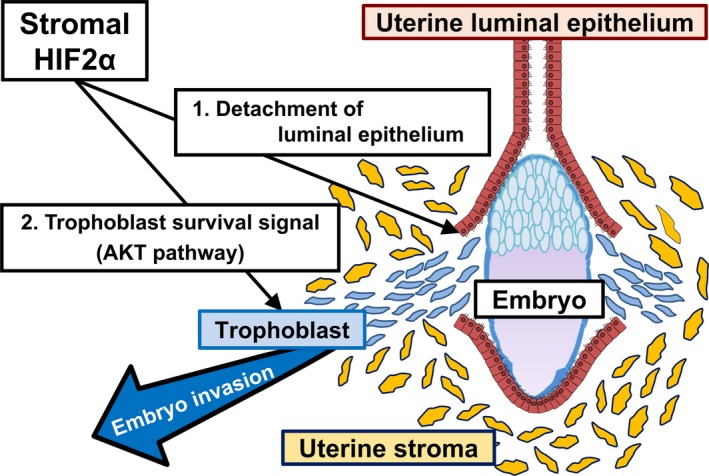
Stromal HIF2α regulates embryo invasion
11. CONCLUSION
The number of women who conceived by IVF‐ET increased markedly for years. To improve fertility rate in IVF‐ET treatment, there remain many issues to be solved, such as recurrent implantation failure despite transfer of good‐quality embryos.6, 60 Implantation failure accounts for a major cause of unexplained infertility, and to date, no efficient treatments exist. Many molecules functioning within the very limited duration are associated with the formation of implantation window, and fundamental research is necessary for elucidating the mechanisms of implantation failure and for establishing its effective treatments. “P4 resistance” is one of the possible mechanisms of implantation failure.12, 19 P4 supplementation treatment for infertility patients is common in humans, and its effectiveness on patients with luteal insufficiency is established.17 However, even P4 supplementation cannot rescue the infertility caused by implantation failure. Accordingly, the present treatment cannot cure patients with severe P4 resistance.
Recent mouse studies revealed that embryo implantation contains multistep processes: uterine receptivity, embryo attachment, and embryo invasion. We consider that implantation failure in humans may be often caused by uterine factors with little relation to P4‐PR signaling involved in each process of embryo implantation such as embryo attachment and embryo invasion, and these patients are out of control of P4 supplementation. We believe that this concept of multistep processes in embryo implantation must help us to develop novel approaches to infertility and contraception.
DISCLOSURES
Conflict of interest: The authors declare that they have no conflict of interest. Human/animal rights: This article does not contain any studies with human and animal subjects performed by the any of the authors.
ACKNOWLEDGEMENTS
This work was supported by JSPS KAKENHI (grant numbers 16H04679, 16H05469, 18K19601, 18K19600, 18H02943), AMED‐Wise (19gk0210021h0001), and Takeda Science Foundation.
Fukui Y, Hirota Y, Matsuo M, et al. Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod Med Biol. 2019;18:234–240. 10.1002/rmb2.12280
REFERENCES
- 1. Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14(6):605‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nachtigall RD. International disparities in access to infertility services. Fertil Steril. 2006;85(4):871‐875. [DOI] [PubMed] [Google Scholar]
- 3. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2‐10. [DOI] [PubMed] [Google Scholar]
- 5. Dey SK, Lim H, Das SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25(3):341‐373. [DOI] [PubMed] [Google Scholar]
- 6. Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185‐199. [DOI] [PubMed] [Google Scholar]
- 7. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Egashira M, Hirota Y. Uterine receptivity and embryo‐uterine interactions in embryo implantation: lessons from mice. Reprod Med Biol. 2013;12(4):127‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345(19):1400‐1408. [DOI] [PubMed] [Google Scholar]
- 10. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266‐2278. [DOI] [PubMed] [Google Scholar]
- 11. Mulac‐Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor‐B isoform. Science. 2000;289(5485):1751‐1754. [DOI] [PubMed] [Google Scholar]
- 12. Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency‐conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117(7):1824‐1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carson DD, Bagchi I, Dey SK, et al. Embryo implantation. Dev Biol. 2000;223(2):217‐237. [DOI] [PubMed] [Google Scholar]
- 14. Haraguchi H, Saito‐Fujita T, Hirota Y, et al. MicroRNA‐200a locally attenuates progesterone signaling in the cervix, preventing embryo implantation. Mol Endocrinol. 2014;28(7):1108‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsumoto L, Hirota Y, Saito‐Fujita T, et al. HIF2α in the uterine stroma permits embryo invasion and luminal epithelium detachment. J Clin Invest. 2018;128(7):3186‐3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Urman B, Yakin K, Balaban B. Recurrent implantation failure in assisted reproduction: how to counsel and manage. A. General considerations and treatment options that may benefit the couple. Reprod Biomed Online. 2005;11(3):371‐381. [DOI] [PubMed] [Google Scholar]
- 17. van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2011;10:CD009154. [DOI] [PubMed] [Google Scholar]
- 18. Hirota Y, Cha J, Dey SK. Revisiting reproduction: prematurity and the puzzle of progesterone resistance. Nat Med. 2010;16(5):529‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tranguch S, Smith DF, Dey SK. Progesterone receptor requires a co‐chaperone for signalling in uterine biology and implantation. Reprod Biomed Online. 2006;13(5):651‐660. [DOI] [PubMed] [Google Scholar]
- 20. Tranguch S, Cheung‐Flynn J, Daikoku T, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102(40):14326‐14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dugan LL, Kim JS, Zhang Y, et al. Differential effects of cAMP in neurons and astrocytes. Role of B‐raf. J Biol Chem. 1999;274(36):25842‐25848. [DOI] [PubMed] [Google Scholar]
- 22. García AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate‐dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10(3):785‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conti L, Sipione S, Magrassi L, et al. Shc signaling in differentiating neural progenitor cells. Nat Neurosci. 2001;4(6):579‐586. [DOI] [PubMed] [Google Scholar]
- 24. Chen J‐F, Mandel EM, Thomson JM, et al. The role of microRNA‐1 and microRNA‐133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daikoku T, Cha J, Sun X, et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell. 2011;21(6):1014‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q, Kannan A, DeMayo FJ, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawagoe J, Li Q, Mussi P, et al. Nuclear receptor coactivator‐6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev Cell. 2012;23(4):858‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ran H, Kong S, Zhang S, et al. Nuclear Shp2 directs normal embryo implantation via facilitating the ERα tyrosine phosphorylation by the Src kinase. Proc Natl Acad Sci USA. 2017;114(18):4816‐4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xin Q, Kong S, Yan J, et al. Polycomb subunit BMI1 determines uterine progesterone responsiveness essential for normal embryo implantation. J Clin Invest. 2018;128(1):175‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mulac‐Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100(17):9744‐9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA. 2003;100(5):2963‐2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diana M, Schettini M, Gallucci M. Evaluation and management of malfunctionings following implantation of the artificial urinary sphincter. Int Surg. 1999;84(3):241‐245. [PubMed] [Google Scholar]
- 33. Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod. 2002;66(2):297‐304. [DOI] [PubMed] [Google Scholar]
- 34. Gregory CW, Wilson EM, Apparao K, et al. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab. 2002;87(6):2960‐2966. [DOI] [PubMed] [Google Scholar]
- 35. Khorram O, Lessey BA. Alterations in expression of endometrial endothelial nitric oxide synthase and alpha(v)beta(3) integrin in women with endometriosis. Fertil Steril. 2002;78(4):860‐864. [DOI] [PubMed] [Google Scholar]
- 36. Mukherjee A, Amato P, Craig‐Allred D, DeMayo FJ, O'Malley BW, Lydon JP. Steroid receptor coactivator 2: an essential coregulator of progestin‐induced uterine and mammary morphogenesis. Ernst Schering Found Symp Proc. 2007;1:55‐76. [DOI] [PubMed] [Google Scholar]
- 37. Mahajan MA, Samuels HH. A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB‐binding protein. Mol Cell Biol. 2000;20(14):5048‐5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee SK, Anzick SL, Choi JE, et al. A nuclear factor, ASC‐2, as a cancer‐amplified transcriptional coactivator essential for ligand‐dependent transactivation by nuclear receptors in vivo. J Biol Chem. 1999;274(48):34283‐34293. [DOI] [PubMed] [Google Scholar]
- 39. Ko L, Cardona GR, Chin WW. Thyroid hormone receptor‐binding protein, an LXXLL motif‐containing protein, functions as a general coactivator. Proc Natl Acad Sci USA. 2000;97(11):6212‐6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caira F, Antonson P, Pelto‐Huikko M, Treuter E, Gustafsson JA. Cloning and characterization of RAP250, a novel nuclear receptor coactivator. J Biol Chem. 2000;275(8):5308‐5317. [DOI] [PubMed] [Google Scholar]
- 41. Cheng JG, Chen JR, Hernandez L, Alvord WG, Stewart CL. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci USA. 2001;98(15):8680‐8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun X, Bartos A, Whitsett JA, Dey SK. Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses. Mol Endocrinol. 2013;27(9):1492‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone‐responsive factor mediating epithelial‐mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245(2):280‐290. [DOI] [PubMed] [Google Scholar]
- 44. Lee K, Jeong JaeWook, Kwak I, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38(10):1204‐1209. [DOI] [PubMed] [Google Scholar]
- 45. Wei Q, Levens ED, Stefansson L, Nieman LK. Indian Hedgehog and its targets in human endometrium: menstrual cycle expression and response to CDB‐2914. J Clin Endocrinol Metab. 2010;95(12):5330‐5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kurihara I, Lee D‐K, Petit FG, et al. COUP‐TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim H‐R, Kim YS, Yoon JA, et al. Estrogen induces EGR1 to fine‐tune its actions on uterine epithelium by controlling PR signaling for successful embryo implantation. FASEB J. 2018;32(3):1184‐1195. [DOI] [PubMed] [Google Scholar]
- 48. Swirnoff AH, Milbrandt J. DNA‐binding specificity of NGFI‐A and related zinc finger transcription factors. Mol Cell Biol. 1995;15(4):2275‐2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhattacharyya S, Fang F, Tourtellotte W, Varga J. Egr‐1: new conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis). J Pathol. 2013;229(2):286‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim H‐R, Kim YS, Yoon JA, et al. Egr1 is rapidly and transiently induced by estrogen and bisphenol A via activation of nuclear estrogen receptor‐dependent ERK1/2 pathway in the uterus. Reprod Toxicol. 2014;50:60‐67. [DOI] [PubMed] [Google Scholar]
- 51. Liang X‐H, Deng W‐B, Li M, et al. Egr1 protein acts downstream of estrogen‐leukemia inhibitory factor (LIF)‐STAT3 pathway and plays a role during implantation through targeting Wnt4. J Biol Chem. 2014;289(34):23534‐23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA‐200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci USA. 2012;109(19):7529‐7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kelleher AM, Peng W, Pru JK, Pru CA, DeMayo FJ, Spencer TE. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc Natl Acad Sci USA. 2017;114(6):E1018‐e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yuan J, Cha J, Deng W, et al. Planar cell polarity signaling in the uterus directs appropriate positioning of the crypt for embryo implantation. Proc Natl Acad Sci USA. 2016;113(50):e8079‐e8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141(12):4365‐4372. [DOI] [PubMed] [Google Scholar]
- 56. Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40(7):871‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80(2):283‐285. [PubMed] [Google Scholar]
- 58. Dengler VL, Galbraith M, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014;49(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Daikoku T, Matsumoto H, Gupta RA, et al. Expression of hypoxia‐inducible factors in the peri‐implantation mouse uterus is regulated in a cell‐specific and ovarian steroid hormone‐dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem. 2003;278(9):7683‐7691. [DOI] [PubMed] [Google Scholar]
- 60. Polanski LT, Baumgarten MN, Quenby S, Brosens J, Campbell BK, Raine‐Fenning NJ. What exactly do we mean by 'recurrent implantation failure'? A systematic review and opinion. Reprod Biomed Online. 2014;28(4):409‐423. [DOI] [PubMed] [Google Scholar]


