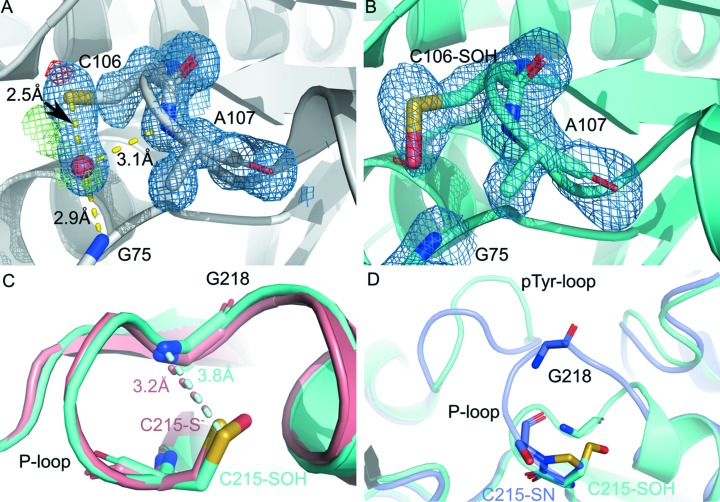
Figure 1.
Cysteine modification in health and disease. (A) Site-specific radiation damage at DJ-1 C106 (PDB ID 2or3, resolution 1.2 Å). A reactive cysteine near a water is a likely prerequisite for hydroxyl radical-mediated, site-specific photo-oxidation of Cys to Cys-SOH. Because of the hydroxyl radical’s extreme reactivity, it will modify the first moiety it contacts, for example a reactive cysteine. A water near C106, stabilized by amides of G75 and A107, is often observed in DJ-1 crystal structures. Difference density, contoured at 3σ, and 2F o − F c density, contoured at 1σ, that is elongated suggests partial oxidation of C106. A water molecule is represented as a red sphere. (B) Cys106-SOH is more fully formed. (PDB ID 3sf8; resolution 1.56 Å). (C) The catalytic cysteine C215 of PTP-1B is located in the P-loop, forming an S-NH hydrogen bond with G218 (salmon, PDB ID 2hnp, resolution 2.8 Å). Oxidation to C215-SOH neutralizes the negative charge of the C215 Sγ thiolate, making it a poorer hydrogen-bond acceptor and lengthening the hydrogen bond by nearly 20% (cyan, PDB ID 1oet, resolution 2.3 Å). (D) Further oxidation to C215-SN disrupts the C215-G218 hydrogen bond, concomitant with large conformational changes propagating to the nearby pTyr recognition loop (slate, PDB ID 1oes, resolution 2.2 Å; the C215-SOH conformation from panel A in cyan). All structures shown in this figure represent data collected at 100 K.