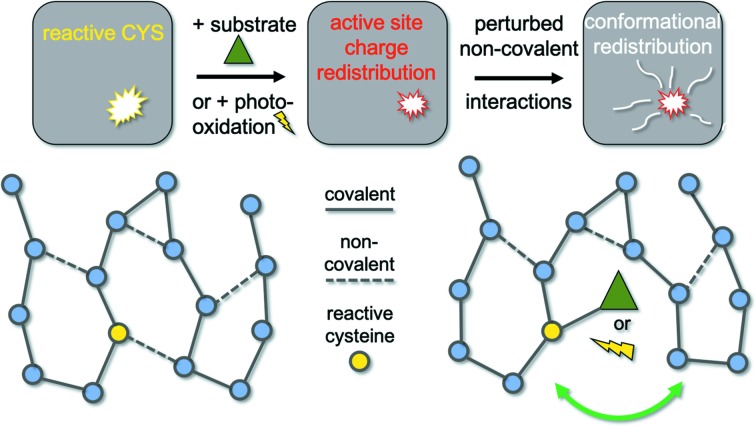
Figure 2.
Cysteine modification reorganizes non-covalent interaction networks similar to on-pathway catalytic intermediates. The graph networks depict a protein structure, with nodes representing residues, solid lines representing covalent bonds, and dashed lines representing non-covalent interactions. A reactive thiolate can be covalently modified through binding a substrate, or by a transient reaction intermediate. The covalent modification neutralizes the charge on the Sγ atom, changing the electrostatic microenvironment of the active site. X-ray induced photooxidation of Cys-S− to Cys-SOH equally results in thiolate charge neutralization. The modified charge of the Sγ acceptor atom considerably weakens or even disrupts any hydrogen bond. This altered non-covalent network can redistribute the protein conformational ensemble well beyond the active site microenvironment.