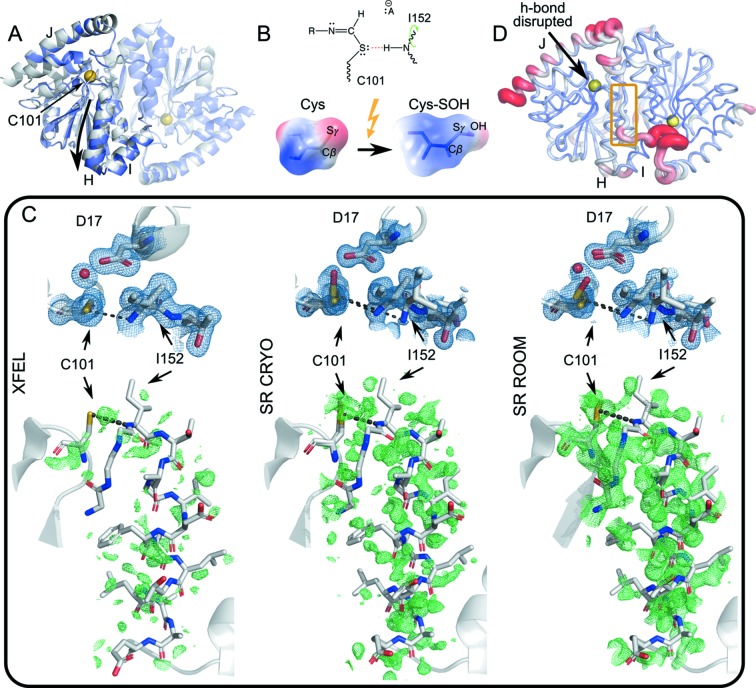
Figure 4.
Conformational dynamics during catalysis in ICH. (A) Homodimeric ICH. The ‘B’ protomer is shown transparent, and the catalytic cysteine Cys101 is indicated with a yellow sphere. The gray cartoon representation corresponds to the unshifted Cys101-S− enzyme. The slate representation is the shifted conformation corresponding to the Cys101-SOH state. While the entire dimer undergoes conformational redistribution, the shift is particularly pronounced for helix H (downward arrow) and linker IJ across the dimer interface. (B) The formation of a thioimidate intermediate neutralizes charge of the C101 Sγ atom (top diagram), weakening the Cys101-Sγ:Ile-NH hydrogen bond (red dashes). Photo-oxidation of Cys101-S− to Cys101-SOH has the same charge-neutralizing effect (bottom), illustrated by surfaces of electrostatic charge. (C) Top panels, 2mF o − DF c electron density maps (blue) of the active site contoured at 1σ. Bottom panels, mF o − DF c difference electron density maps (green) around helix H, phased with the XFEL conformer (PDB ID 6npq, resolution 1.6 Å), contoured at 2σ. The (unshifted) atom positions in the ‘SR-CRYO’ (PDB ID 6nja, resolution 1.05 Å) and ‘SR-ROOM’ (PDB ID 6ni6, resolution 1.2 Å) bottom panels are virtually identical to those of the XFEL conformer on the left. The conformational ensemble shifts as Cys101 is more fully oxidized, manifested as shifting occupancies of the Cys-S− and Cys-SOH states, from XFEL to synchrotron (SR) X-ray irradiation at room temperature. (D) ICH protein conformational dynamics most affected by the disruption of the Cys101-Sγ:Ile-NH hydrogen bond. We selected ten low free energy motion modes from a KFA analysis that overlapped least for the Cys-S− enzyme with the Sγ-NH hydrogen bond intact and without the Sγ-NH hydrogen bond in one of the dimer subunits. These motion modes are most affected by the change in hydrogen-bonding network. The figure shows the root-mean-square fluctuations when these motion modes are sampled. The IJ linker from the opposite dimer subunit appears strongly affected by the disruption (yellow box).