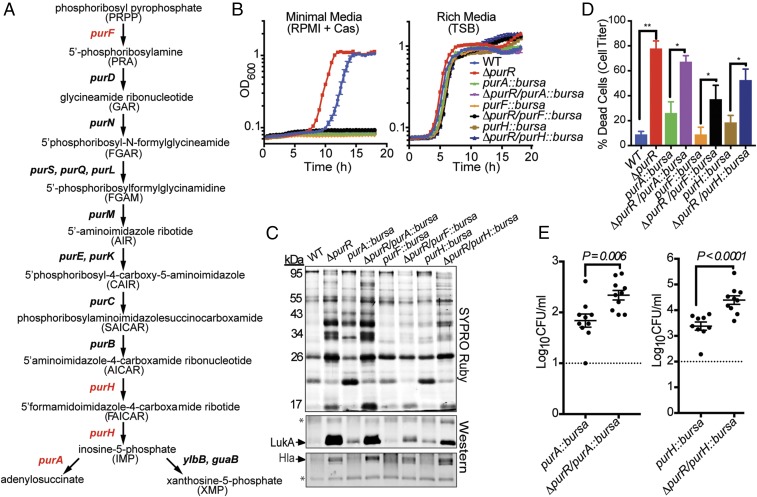
Fig. 3.
purR mutant is hypervirulent independent of purine biosynthesis. (A) The purine biosynthesis pathway in S. aureus, as adapted from Mongodin et al. (40). Genes highlighted in red were mutated in our study. (B) Mutants for the purine biosynthesis genes purA, purF, and purH cannot grow in minimal media (RPMI + casamino acids) in either the wild-type or purR mutant background (n = 3 replicates per strain). These mutations do not prevent the strains from growing in rich media (TSB) (n = 3 replicates per strain). (C) Supernatants obtained from purine biosynthesis mutants in the purR mutant background generate elevated levels of secreted proteins, including LukAB and Hla. Asterisks indicate loading controls, which are nonspecific bands recognized by the antibodies. (D) Ten percent culture filtrates obtained from purine biosynthesis mutants in the purR mutant background are more cytotoxic toward primary human PMNs than supernatants obtained from their parental strains (n = 5–8 donors per strain). Statistical analysis was determined using a two-tailed Mann–Whitney U test. Error bars indicate SEM. *P < 0.05, **P < 0.01. (E) Kidneys of mice infected i.v. (2.5 × 107 CFUs) with either ΔpurR/purA::bursa or ΔpurR/purH::bursa for 20 h demonstrate higher bacterial burdens than mice infected with the respective parental strains of these mutants, purA::bursa and purH::bursa (n = 10 mice per strain). Dotted lines represent the limit of detection in these organs. Statistical significance was calculated using a two-tailed t test.