Fig. 1.
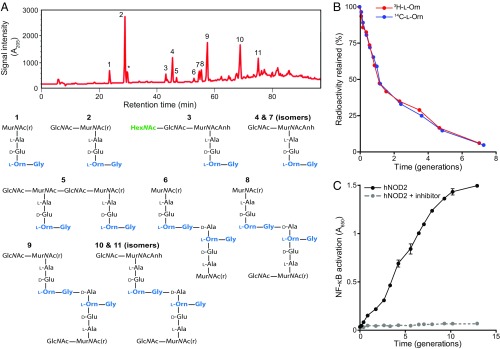
B. burgdorferi sheds muropeptides into its extracellular environment. (A, Top) Chromatogram of cellosyl-digested and reduced PGBb isolated from B. burgdorferi B31. Numbers correspond to the identified chemical species shown below. The asterisk indicates an unidentified species (SI Appendix, Table S1). Analysis performed on three separate preparations produced highly similar chromatograms. (A, Bottom) Chemical composition of muropeptides in peaks shown in the chromatogram. Muropeptide identification was accomplished by MS. MurNAc(r) and Anh indicate N-acetylmuramitol and 1,6-anhydro group, respectively. (B) Plot showing PG turnover over multiple generations in B. burgdorferi grown in vitro. PGBb was pulse-radiolabeled by incubating cells in medium containing 7.5 µCi/mL of 3H- or 14C-l-Orn for 48 h. Cells were then washed to remove unincorporated isotope, and outgrowth was tracked in complete BSK II medium lacking radioactive l-Orn. At each time point, the same volume of batch culture was removed, bacterial density was determined, and PGBb was purified for quantification of its radioactivity per volume equivalent. The retained radioactivity was then plotted as a percentage of total radioactivity in the PG at time 0 (i.e., start of outgrowth). (C) Muropeptide accumulation in the culture medium. Cultures of B. burgdorferi (5 × 107 cells per milliliter) were diluted to a starting density of 104 cells per milliliter and monitored for muropeptide release during growth in complete BSK II medium (lacking phenol red) using an hNOD2 reporter cell line in the presence or absence of the RIP2 inhibitor gefitinib. NF-κB activity (absorbance at 650 nm) provides a measure of NOD2-specific muropeptide levels present in the culture medium samples collected at the indicated time points. Shown are the mean and SD of NF-κB activation for two biological replicates at each time point.