Abstract
Rationale:
Pulmonary arterial hypertension (PAH) is a deadly disease with no cure. Alternate conversion of Angiotensin II to Angiotensin-(1–7) (Ang-(1–7)) by angiotensin converting enzyme 2 (ACE2) resulting in Mas receptor (Mas1) activation improves rodent models of PAH. Effects of rhACE2 in human PAH are unknown.
Objectives:
To determine the effects of rhACE2 in PAH.
Methods:
We defined the molecular effects of Mas1 activation using porcine pulmonary arteries, measured AngII/Ang-(1–7) levels in human PAH and conducted a phase IIa open-label pilot study of a single infusion of rhACE2 (GSK2586881, 0.2 mg/kg or 0.4mg/kg IV, NCT01884051).
Results:
Superoxide dismutase 2 (SOD2) and inflammatory gene expression were identified as markers of Mas1 activation. After confirming reduced plasma ACE2 activity in human PAH, 5 patients were enrolled in the trial. GSK2586881 was well tolerated with significant improvement in cardiac output and pulmonary vascular resistance. GSK2586881 infusion was associated with reduced plasma markers of inflammation within 2–4 hours and increased SOD2 plasma protein at two weeks.
Conclusions:
PAH is characterized by reduced ACE2 activity. Augmentation of ACE2 in a pilot study was well-tolerated, associated with improved pulmonary hemodynamics and reduced markers of oxidant and inflammatory mediators. Targeting this pathway may be beneficial in human PAH.
Keywords: Pulmonary arterial hypertension, drug trial, hemodynamics, ACE2
Introduction:
Pulmonary arterial hypertension (PAH) is a highly morbid disease that primarily affects young women with progressive pulmonary vascular obliteration resulting in right heart failure and death. While much progress has been made in improving outcomes in PAH, therapies are imperfect and there is presently no cure. Newer, more effective treatments are needed that address key disrupted pathways in PAH.
Although the renin-angiotensin-aldosterone system is known to be activated in PAH1–4, trials of angiotensin converting enzyme (ACE) inhibitors in this disease have not demonstrated benefit5, 6,7, 8 and although AT-1 receptor antagonism has benefit in the monocrotaline rat model of pulmonary hypertension, this drug class has not been trialed in humans with PAH1. The observation has led to the hypothesis that alternate hydrolysis of AngII to Ang-(1–7) via angiotensin converting enzyme 2 (ACE2) may be a more effective therapeutic intervention. Ang-(1–7) activates the Mas receptor (Mas1), which is present on endothelial cells and has vasodilatory, anti-inflammatory and anti-fibrotic effects9, 10, functionally antagonizing the effects of AT1 receptor stimulation11–13. Finally, activation of the ACE2-Ang-(1–7) axis reduces oxidant stress in diabetes mellitus14, suggesting impact on pathways of relevance to PAH15–17. Thus the ACE2-Ang-(1–7)-Mas1 axis may be a promising therapeutic pathway in PAH.
We and others have demonstrated that both infusion of ACE2 and direct activation of Mas1 ameliorate rodent models of PAH18–23 likely through improved cytoskeletal function, which is consistent with prior work on ACE224. Further, the ACE2-Ang-(1–7) axis has been studied in a right ventricular (RV) failure model in which ACE2 peptide administration resulted in reduced RV hypertrophy and fibrosis improved function19, suggesting potentially beneficial effects on both the pulmonary vasculature and also RV load stress responses. In human patients, there are no FDA-approved mechanisms to stimulate Mas1 and there are no direct Mas1 agonists approved for human use. ACE2 enzymatic activity can be augmented by administration of an intravenous formulation of soluble recombinant form of the naturally occurring enzyme rhACE2 with existing safety data in healthy volunteers and ARDS (GSK2586881, NCT01597635)25.
We tested the hypotheses that ACE2 activity is reduced in human PAH compared with healthy controls and that short term ACE2 administration may be safe in a proof of concept pilot study of GSK2586881 in PAH patients. We further sought to identify short-term markers of Mas1 activation, suggesting molecular drug effect, which may facilitate future studies of Mas1 activation in PAH.
Methods:
Animal Experiments
All animal studies were approved by the Vanderbilt University Medical Center (VUMC) Institutional Animal Care and Use Committee.
Pulmonary arterial isolation and cannulation
Pulmonary arteries, 80 to 300 μm diameter were dissected from portions of the piglet lungs with previously described methods26. Please see online supplement for full details. Please see online supplement for details of RNASeq experiments performed as previously reported27, 28.
Observational Studies of ACE2 in PAH patients
Human studies were approved by the VUMC IRB (#s 9401 and 151388) and registered at clinicaltrials.gov ( NCT01884051). All patients gave written informed consent prior to inclusion in the study. Idiopathic and heritable PAH patients, aged 18 years or older, defined according to standard criteria by expert clinicians, according to standard criteria were included29. Please see online supplement for details of RAS peptide measurement and aptamer-based superoxide dismutase (SOD2) protein measurement.
Pilot Trial of GSK2586881
The study was a phase I dose-escalation, open-label proof of concept study in patients with idiopathic or heritable PAH, functional class I-III. The primary endpoint was safety, with secondary endpoints of change in disease biomarkers (brain natriuretic peptide (BNP), AngII/Ang-(1–7) ratio, SOD2 activity and nitrotyrosine activity), systemic and pulmonary hemodynamics and echocardiographic metrics of PAH including right ventricular (RV) function. We planned three patients at 0.2mg/kg based on prior safety data with an escalation to 0.4mg/kg if no dose limiting toxicity occurred. Please see online supplement for detailed inclusion and exclusion criteria.
Patients were recruited from the pulmonary hypertension clinic at VUMC from 3/2016–12/2016. Study procedures are outlined elsewhere (Supplemental Table 3). Briefly, patients underwent right heart catheterization. Hemodynamics were recorded one hour prior to drug administration (−1H), immediately prior to drug administration (0) and one, two and four hours after administration. Patients were observed overnight with six-minute walk test and echocardiography measured 24 hours after drug administration. Patients returned two weeks after drug infusion for safety assessment, 6MWD and clinical evaluation. Safety endpoints assessed included change in functional class, development of right heart failure signs or symptoms, six minute walk distance, plasma electrolytes and markers of renal function, complete blood count, echocardiography-derived right ventricular function.
Please see online supplement for details of RAS peptide measurement, SOD2 ELISA, nitrotyrosine dot blot assay, cytokine luminex assay, and isoprostane and isofuran measurement
Statistical Analysis
Continuous variables of demographic data are reported as mean ± standard deviation. The Wilcoxon rank-sum test and the Mann Whitney U test were used to compare differences between groups. Categorical variables were compared between groups using the chi-square test or Fisher exact test. A p value of < 0.05 was considered statistically significant. Data from the pilot study were compared using paired two-tailed t test. Statistical analyses were performed using Prism 5.0 software (Graph Pad Software Inc, La Jolla, CA) and R 3.0.1.
Results:
ACE2 activity in human PAH:
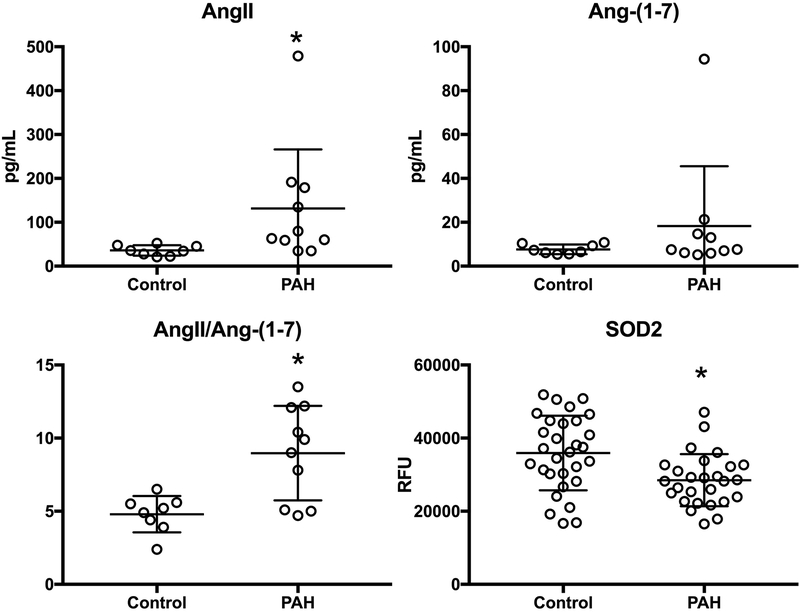
We first tested the hypothesis that ACE2 activity is suppressed in human PAH patients compared to controls. We enrolled 11 consecutive heritable or idiopathic PAH patients and eight healthy controls (2 males, 6 females, Supplementary Table 1). We measured AngII and Ang-(1–7) in plasma and found a significant decrease in ACE2 activity as reflected by the AngII/Ang-(1–7) ratio in PAH compared with controls (p=0.01, Figure 1). This difference was driven by an increase in plasma AngII, that was nearly four fold higher in PAH than control (p<0.003) with a less significant difference in Ang-(1–7) levels in PAH vs. control.
Figure 1. Evidence of insufficient ACE2 activity in human PAH.
Plasma Angiotensin II (AngII) and Angiotensin-(1–7) levels were measured in 11 PAH patients and 8 healthy controls as a marker of ACE2 activity. Log transformed values are normally distributed by Shapiro-Wilk W Test for each variable and the ratio, allowing parametric testing. AngII was increased in PAH (*p<0.003) and AngII/Ang-(1–7) ratio was increased (*p=0.01) suggesting reduced conversion of AngII to Ang-(1–7) by ACE2 in the plasma of PAH patients. SOD2 protein level in plasma was measured by aptamer-based proteomic assay in 25 PAH patients and 26 age-, sex-, and BMI-matched controls. SOD2 protein was reduced in PAH (*p=0.002). RFU = relative fluorescent units.
Biochemical effects of Mas1 activation:
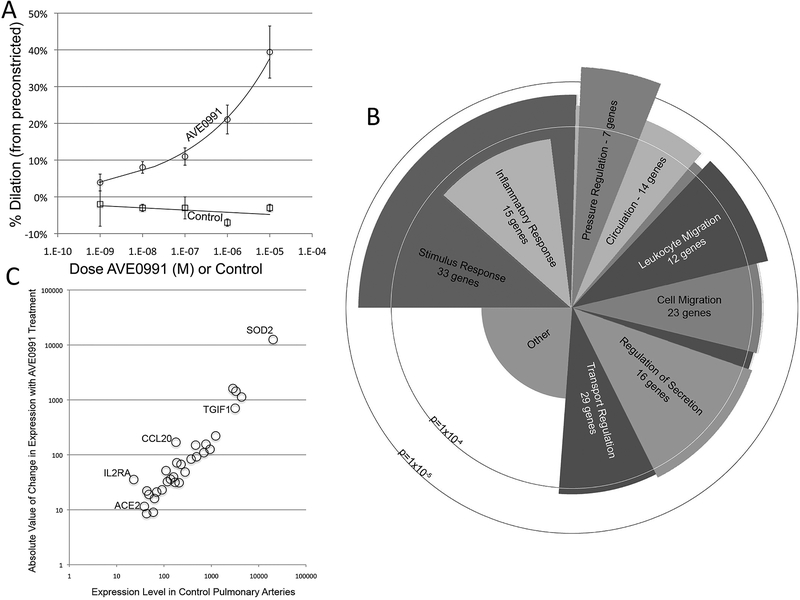
We next sought to define the molecular consequences of Mas1 activation. Because ACE2 requires AngII as substrate and Ang-(1–7) has a short half life, the use of ACE2 in isolated artery studies is challenging. Subsequently we used AVE0991, a direct Mas1 agonist, to determine if Mas1 activation results in pulmonary artery vasodilation (Figure 2A). Using AVE0991 in porcine pulmonary arteries preconstricted with ET-130, we found a dose-dependent increase in percent dilation as a function of AVE0991, demonstrating that Mas1 activation has similar physiologic effects in this model as seen in rodent models18, 20, 21.
Figure 2. Acute molecular effects of Mas1 activation in a porcine pulmonary hypertension model.
AVEO991, a direct Mas receptor agonist, was administered to pig arteries pre-constricted with ET-1. A. There was a dose-dependent increase in percent dilation with AVE0991 administration. No dilation was seen with exposure to a biologically irrelevant control compound. B. RNA was isolated from pig arteries with and without AVE0991 exposure and RNASequencing performed. Gene ontology analysis demonstrated significant differences in gene expression in several pathways including inflammatory responses (p<1×10−4), cell and leukocyte migration (p<1×10−4) and pressure regulation (p<1×10−5). C. When absolute change in gene expression with drug exposure was plotted as dependent on expression level in control arteries, SOD2 expression was strongly up-regulated in response to Mas1 activation. N=8 vessels.
To determine the acute changes in gene expression as a result of Mas1 activation in this isolated artery model, we isolated RNA from arteries exposed to AVE0991 and controls and performed RNASeq. Significantly changed genes are presented in Supplementary Table 2. Using a gene ontology analysis approach (Figure 2B), we found significant changes in several pathways including genes associated with pressure regulation (p<1×10−5), inflammatory responses (p<1×10−4) and cell migration (p<1×10−4), again suggesting potential effects of Mas1 activation on inflammation and cytoskeletal function. In order to define a potential marker for Mas1 activation in the pulmonary artery, we compared pulmonary arterial gene expression levels before and after AVEO991 (Figure 2C). Several genes were up-regulated as a result of AVE0991 exposure, including TGIF-1, CCL2 and SOD2. CCL2 is known to be of relevance to PAH, suggesting potential relevance to PAH31. SOD2, a mitochondrial protein catalyzing production of hydrogen peroxide and oxygen from superoxide, was an attractive marker based on prior publications32–34 and a known effect of Mas1 activation in reducing oxidative stress in rodent models of diabetes14, suggesting potential biologic plausibility and also it’s significant upregulation after AVE0991 exposure. Validation using qPCR in pulmonary arteries can be found in Supplemental Figure 1. Further, SOD2 expression was high in the control pulmonary arteries and demonstrated marked increase in gene expression after Mas1 activation, suggesting it may be a biologically important and sensitive marker of increased ACE2 activity.
SOD2 in human PAH:
We next sought to determine if SOD2 levels were different in human PAH and control patients. We used an aptamer-based proteomic profiling approach to measure relative plasma SOD2 protein content in fasting peripheral blood in 25 PAH patients (mean age 49.5(13.2), BMI 29.8 (8.2), 3 males), and 26 matched controls (mean age 46.7 (11.8), BMI 29.1 (6.7), 5 males), Figure 1D. We found levels of SOD2 protein were approximately 25% lower in PAH plasma compared with controls (28,489 (7112) vs. 35,930 (10,202) RFU, p= 0.002).
Pilot trial of GSK2586881 in PAH:
We next sought to determine the safety and potential acute hemodynamic and biochemical effects of a single IV infusion of GSK2586881 administration in human PAH patients (see Supplementary Table 3 for details of the protocol). We enrolled five PAH patients and used two different GSK2586881 doses (patients 1–3, 0.2mg/kg and patients 4 and 5, 0.4mg/kg, Table 1). All patients were receiving at least two PAH-directed therapies including prostaglandin pathway treatments. All patients completed the study protocol and there were no serious adverse effects with study drug administration. One patient experienced dizziness without change in vital signs (patient 1) within one hour of drug infusion and one patient reported leg tingling during the overnight observation period (patient 5) that did not require intervention.
Table 1.
Characteristics of patients enrolled in GSK2586881 trial
| Patient | Age (years) | Sex | BMI (kg/m2) | PAH type | Disease Duration (months) | Current Therapy | Co-morbid Disease |
|---|---|---|---|---|---|---|---|
| 1 | 28 | F | 27.5 | Idiopathic | 13 | PP, PDE5I | none |
| 2 | 39 | F | 27.8 | Idiopathic | 60 | PP, PDE5I, ERA | none |
| 3 | 57 | M | 32.5 | Idiopathic | 91 | S,PDE5I | DM2, HTN, HLD |
| 4 | 41 | F | 27.4 | Heritable | 92 | T, PDE5I, ERA | none |
| 5 | 68 | F | 28.3 | Idiopathic | 65 | PP, PDE5I, ERA | DM2, HTN |
Patients 1, 2 and 3 received 0.2mg/kg. Patient 4 and 5 received 0.4 mg/kg GSK2586881. PP=parenteral prostaglandin, PDE5I = phosphodiesterase type 5 inhibitor, ERA = endothelin receptor antagonist, S=selexipag, T=inhaled treprostinil, DM2 = type 2 diabetes mellitus, HTN = systemic hypertension, HLD = hyperlipidemia
There was no statistical or clinically significant change in other safety parameters including six-minute walk distance (Supplementary Table 4). All patients were functional class II at baseline and 4 were functional class II at 2 week follow up, one patient was functional class I. With the exception of a small but statistically significant decrease in creatinine from baseline to 24 hours (1.03 ± 0.4mg/dL vs. 0.99 ± 0.4mg/dL, p=0.03), there were no significant changes in laboratory values (data not shown). There were no differences in echocardiographic metrics of left ventricular or RV function at 24 hours (Supplementary Table 5). No antidrug antibodies were detected at two weeks in any patient (data not shown). Pharmacokinetic profiles are listed in Supplemental Table 6.
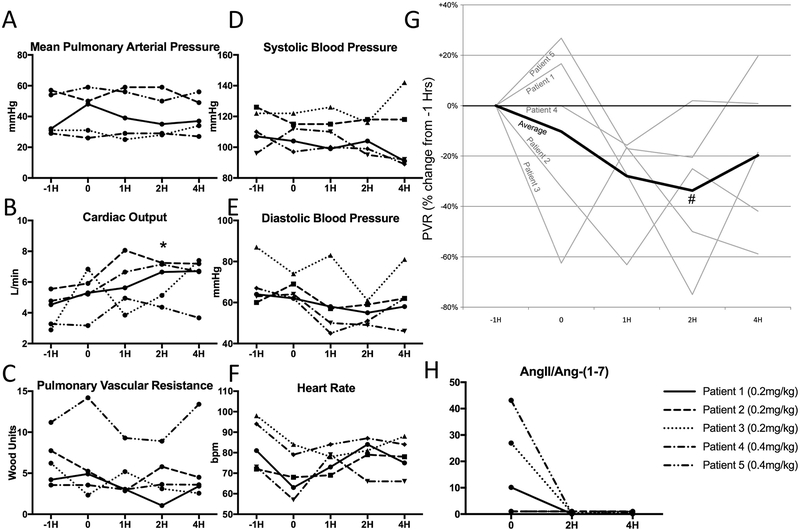
A single dose of GSK2586881 had no effect on mean pulmonary arterial pressure (mPAP) (Supplementary Table 7, Figure 3A); however we did observe a statistically significant increase in cardiac output (5.3 ± 1.4L/min vs. 6.1 ± 1.3L/min, p=0.008) four hours after study drug infusion, representing an average increase of 40% (Figure 3B). Pulmonary vascular resistance (PVR) would be expected to decrease when cardiac ouput increases without a change in mPAP. We observed substantial variability in PVR with low numbers of enrollees, but when comparing combined time points prior to drug administration with after infusion in normalized PVR, we found a statistically significant decrease in PVR, reaching a nadir two hours after infusion (Figure 3G, p<0.05). There were no significant effects on systemic hemodynamics (Figure 3D–F).
Figure 3. Pulmonary and systemic hemodynamic effects of intravenous GSK2586881.
A. A pulmonary artery catheter was placed and hemodynamics measured (−1H). Just prior to GSK2586881 exposure, pulmonary hemodynamics were recorded again (0) and then 1 hour (1H), 2 hours (2H) and 4 hours (4H) after drug administration, n=5. There was no significant change in mean pulmonary arterial pressure. B. There was a significant increase in cardiac output 2 hours after drug administration (*p=0.008). C-F. Individual raw data on pulmonary vascular resistance, systolic blood pressure, diastolic blood pressure and heart rate. G. Percent (%) change in pulmonary vascular resistance (PVR) showed a significant decrease when comparing time points before drug administration (−1H and 0) with those after (1H, 2H and 4H), # = p<0.05. H. Angiotensin II (AngII) and Angiotensin-(1–7) (Ang-(1–7)) levels were measured just prior to drug (0) and 2 hours (2H) and 4 hours (4H) after drug exposure. In the 3 patients with elevation in this ratio at baseline, there was a significant decrease in the ratio, suggesting effective ACE2 augmentation with administration of GSK2586881 (p=0.009 and 0.008 vs. 0 at 2H and 4H).
Plasma AngII and Ang-(1–7) levels were measured as a marker of clinical pharmacology. A decrease in the ratio of AngII/Ang-(1–7) suggests increased activity of ACE2 and thereby effective augmentation by the study drug. There was significant variability in the ratio of AngII/Ang-(1–7) ratio at baseline (Figure 3H). Three patients had substantial elevations in this ratio at baseline and, in these patients, there was a reduction to nearly undetectable ratio with drug administration (26.5 ± 16.5 vs. 0.2 ± 0.2, p=0.009) that was sustained at four hours (0.3 ± 0.2, p=0.008). Full details of change in RAS peptides are in Supplemental Figure 3.
Effect of GSK2586881 on SOD2 in human PAH:
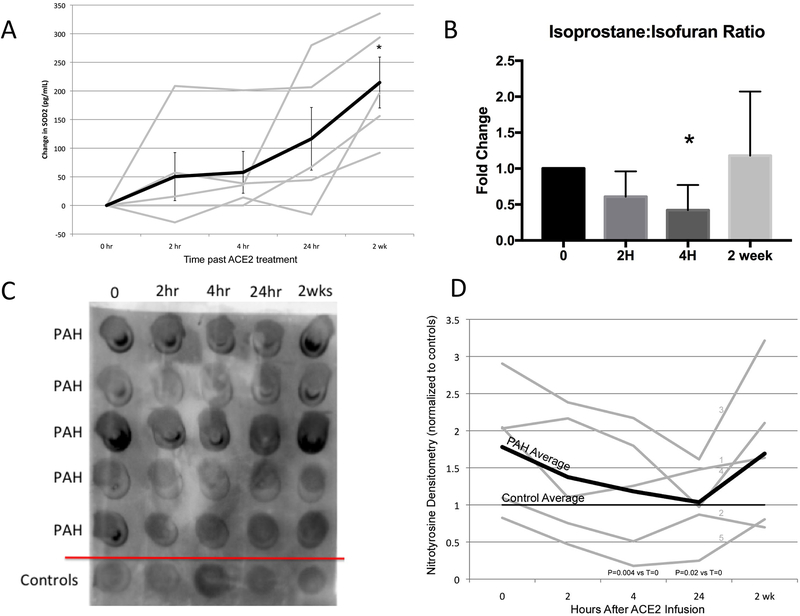
As our ex vivo animal model had shown induction of SOD2 expression with Mas1 activation, we tested the hypothesis that SOD2 protein content in PAH patient plasma would be induced by GSK2586881 administration (Figure 4A). Using an ELISA assay, we found that there was significant induction of plasma SOD2 protein levels by two weeks (p=0.009 vs. baseline values) suggesting induction in the enzymatic activity by GSK2586881, corroborating our findings in the animal model and indicating that this may be a marker of Mas1 activation in human PAH.
Figure 4. Effect of GSK2586881 on markers of oxidant stress.
A. Plasma SOD2 protein levels were measured by ELISA assay at just prior to drug administration (0), and 2 hour (2 hr), 4 hour (4 hr), 24 hours (24 hr) and 2 weeks (2 wk) after drug administration, n=5. There is a significant increase in plasma SOD2 levels at two weeks (*p=0.009). B. We measured plasma isoprostanes and isofurans as markers of oxidant stress. Isofurans are lipid peroxidation products similar to isoprostanes, but are formed in times of oxygen availability. There was a decrease in the isoprostanes:isofuran ratio (*p<0.05), suggesting improved oxygenation. C. Nitrotyrosine dot blot assay from the plasma of 5 healthy controls and study enrollees (PAH) and quantification of densitometry D. In PAH patients, there was a decrease in plasma nitrotyrosine levels to that of controls by four hours (p=0.004) that was sustained at 24 hours (p=0.02). Please see Online Supplement Figure 1 for full original blot.
Effects of GSK2586881 on plasma markers of inflammation:
Our animal microarray data further suggested that Mas1 activation potentially acts through altering the pattern of pro-inflammatory gene expression pattern. We next tested the hypothesis that this improvement would occur in human PAH patients after acute GSK2586881 administration. As markers of reduced pro-inflammatory signaling, we measured a plasma cytokine array and markers of oxidant stress. We utilized a luminex platform to assess the effect of GSK2586881 on plasma cytokines (Table 2). Consistent with prior reports35, there were increased levels in 6/9 measured cytokines at time point 0 compared with controls (p<0.05 for IL-10, IL-1β, TNFα, IL-13, IL-8 and IL-4). With GSK2586881 administration, there was suppression of measured cytokines including IL-10, IL-1β, IL-2 and TNFα that could be detected as early as two hours after drug administration and was associated with sustained anti-inflammatory effects with reduced levels of IL-1β, IL-6, IL-8 and TNFα at two weeks (p<0.05). These data collectively suggested a broad suppression of inflammatory cytokines with rhACE2 administration in PAH.
Table 2.
Effect of GSK2586881 on Plasma Cytokines
| Control | 0 | 2H | 4H | 24H | 2WK | |
|---|---|---|---|---|---|---|
| IFNγ | 8.4±5.1 | 17.1±10.9 | 14.9±12.9 | 15.4*±9.8 | 20.5±17.8 | 15.7±11.2 |
| IL-10 | 6.6±5.5 | 15.9$±5.3 | 12.0*±3.8 | 13.8±6.6 | 19.1±10.4 | 13.6±9.1 |
| IL-13 | 11.6±9.5 | 39.1$±15.0 | 30.3±14.3 | 32.9*±14.2 | 41.0±22.2 | 34.9±18.6 |
| IL-1β | 1.3±0.5 | 3.8$±2.0 | 2.6*±1.8 | 3.0#±1.7 | 4.3±3.2 | 3.0*±1.8 |
| IL-2 | 3.1±1.4 | 6.3±3.0 | 4.8±3.0 | 5.6*±2.8 | 7.1±5.2 | 5.6±2.6 |
| IL-4 | 14.0±11.4 | 42.8$±26.5 | 34.5±23.3 | 35.7±19.7 | 50.0±35.7 | 36.9±27.3 |
| IL-6 | 2.5±1.8 | 4.6±1.8 | 4.0±1.9 | 9.2±6.7 | 6.4±6.4 | 3.3#±1.5 |
| IL-8 | 5.0±1.6 | 8.6$±1.5 | 7.5±2.5 | 8.5±1.6 | 9.2±2.9 | 6.9*±1.4 |
| TNFα | 4.8±3.2 | 9.1$±1.8 | 5.6*±3.0 | 6.8#±1.9 | 9.6±5.3 | 5.2*±1.5 |
Mean cytokine levels (pg/ml) for five healthy controls and five PAH patients treated with ACE2. 0=immediately before drug infusion, 2H= 2 hours after infusion, 4H=4 hours after infusion, 24H=24 hours after infusion, 2WK = 2 weeks after infusion.
=p<0.05 vs. control by paired t-test with normalized data,
=p<0.05 vs pretreatment (time point 0) by paired t-test with normalized data,
= p<0.01 vs pretreatment (time point 0) by paired t-test with normalized data.
Effect of ACE2 on oxidant stress:
Plasma levels of oxidized lipids are known to be increased in PAH as a marker of oxidant stress16, 17. Isofurans are lipid peroxidation products similar to isoprostanes, but isofurans exhibit favored formation in the presence of increased oxygen availability36. The ratio of isoprostanes to isofurans is a useful index of oxygen availability. We measured isoprostanes and isofurans in the plasma of our trial participants and found a significant decrease in the isoprostane to isofuran ratio four hours after GSK2586881 infusion (Figure 4B, p<0.05).
To further assess the effect of GSK2586881 on markers of oxidant stress, we quantified plasma 3-nitrotyrosine levels by dot blot assay on five healthy controls and on our study participants (Figure 4C and D). We found that nitrotyrosine was increased in PAH patients compared to controls (p=0.02) at baseline. With GSK2586881 administration, plasma nitrotyrosine levels fell in PAH patients at both four and 24 hours (p =0.02 and p=0.002, respectively). At the 24-hour time point, PAH nitrotyrosine levels were no different from controls after GSK2586881 administration, suggesting a significant reduction in whole body oxidant stress.
Discussion:
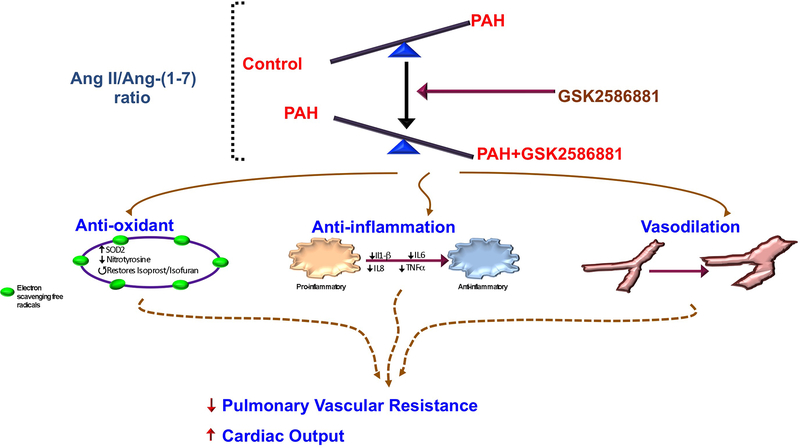
Our study sought to 1) demonstrate reduced ACE2 activity in human PAH, 2) define robust readouts of Mas1 agonism in animal models and determine if these readouts are potentially relevant in human PAH and 3) perform a pilot trial of GSK2586881 in PAH patients to determine if this biologic is potentially safe in human PAH and may demonstrate either hemodynamic or biochemical efficacy. We aimed to derive a marker of Mas1 agonism in our pig pulmonary artery model that was of relevance to PAH and to test the hemodynamic and pharmacodynamics effects of GSK2586881 in human PAH patients. These experiments demonstrate that rhACE2 administration in human PAH may have potential biochemical and hemodynamic efficacy through Mas1 activation. A visual summary of the findings and interpretations are demonstrated in Figure 5.
Figure 5. Schematic of study findings.
PAH: pulmonary arterial hypertension; AngII: angiotensin II; Ang-(1–7): angiotensin-(1–7); SOD2: superoxide dismutase 2; IP: isoprostane; IF: isofuran; IL: interleukin; TNF: tumour necrosis factor.
Given compelling preclinical data in rodent models of PAH18–21 and pathology specimens demonstrating up regulation of the renin-angiotensin system (RAS) in PAH3, 4, interventions on the RAS pathway would seem likely to have benefit. After demonstrating relevance of this pathway to human PAH and defining inflammatory markers and SOD2 as potential markers of drug effect relevant to pulmonary vascular disease, we proceeded with a pilot study to assess safety of GSK2586881 in PAH, restricting entry to idiopathic and heritable PAH as our rodent model experiments were most relevant to these PAH sub-groups18. We enrolled five patients with advanced disease, as evidenced by multiple PAH-directed therapies including those targeting the prostacyclin pathway. We did not identify any safety concerns although we enrolled relatively low numbers of patients. Even in this small study there were potential hemodynamic markers of efficacy, including improved cardiac output and PVR. Further, there was additional biochemical data supporting improved cardiac output: the drop in creatinine may be consistent with improved renal perfusion and reduced isoprostane:isofuran ratio may indicate improved oxygen delivery to the systemic circulation.
A major hindrance to drug development in PAH is the lack of effective biological markers of drug efficacy. We sought to overcome this limitation by first developing markers of drug effect in relevant tissue, the pig pulmonary artery. We identified SOD2 and inflammatory pathway genes as having robust changes in response to AVE0991 exposure. SOD2 plays a role in animal models of PAH, though data in human PAH are limited32–34. Using proteomic data, we showed that SOD2 protein was reduced in PAH plasma compared with controls, suggesting potential relevance in humans with detectable protein levels. Human and rodent data show a role for inflammation in PAH35, 37, 38, thus we tested cytokine levels in our own PAH population and found elevations in several cytokines in plasma compared to controls. After demonstrating the potential relevance of SOD2 and inflammation in PAH, we next tested the potential effect of Mas receptor activation on these markers in human PAH. We found that rhACE2 augmentation resulted in similar effects in the plasma of PAH patients as direct Mas receptor activation in pig arteries. These findings correlated with improved cardiac output at the time of maximum suppression of plasma cytokines and maximum reduction in plasma nitrotyrosine levels, a downstream marker of SOD2 activity. SOD2 levels rose at a later timepoint in the plasma, but this was expected as SOD2 is located in the mitochondria and cell turnover would be required prior to detection of a change in plasma levels. Although AngII/Ang-(1–7) ratio also reflected effective increase in plasma ACE2 activity, these changes in SOD2, nitrotyrosine and cytokines are likely more reflective of activation of Mas1 activation with changes in downstream signaling. Indeed, prior work has demonstrated that Ang-(1–7) inhibits leukocyte migration and cytokine expression39, 40. While inflammation and SOD2 are potential markers of drug effect, their levels may be altered by other exposures and pathology, making the relative change with drug exposure particularly of interest. It is possible that future drug trials in this pathway may use these markers of Mas1 agonism as early read outs of biochemical efficacy in adaptive trial designs or to define patients most likely to benefit from these interventions.
Oxidant stress has long been implicated in PAH;33, 41–46. Whole body markers of oxidant stress include F2-isoprostanes, formed through free-radical peroxidation of essential fatty acids, and nitrotyrosine, a product of tyrosine nitration by reactive nitrogen species. Both nitrotyrosine and isoprostanes have been implicated in PAH17, 44, 47. Isofurans, in contrast, are non-enzymatic compounds formed through free radical peroxidation of arachidonic acid and indicate increased production of oxidants at the level of the mitochondria and thus require oxygen to produce. We hypothesized that increased Mas1 signaling would reduce total burden of oxidant stress in PAH patients. We found reduction in plasma nitrotyrosine by dot blot assay, consistent with this hypothesis. There was a significant decrease in the plasma isoprostane:isofuran ratio at four hours that correlated with the time point of greatest improvement in cardiac output, suggesting increased delivery of oxygen to tissues and potentially greater oxygen-dependent respiration reflected in reduction in this ratio although this hypothesis requires further confirmation. Overall, the data were reassuring that there was not an increase, and rather a decrease, in total body oxidant stress as measured by nitrotyrosine levels that showed reduction to normal levels at 4 and 24 hours after drug infusion.
Our study is limited by the low number of trial participants, however our intention was to perform a proof of concept study and to identify potential markers of drug efficacy that can be used in a larger trial. Our RNA expression markers of AVE0991 effects in the pig vasculature were reduced by the known transcriptome of pigs which is limited compared with humans, thus there may be more significantly changed transcripts that could not be identified by our methodology; nonetheless we were able to detect robust readouts. There are other members of the RAS peptide family that would be informative regarding ACE2 activity including Ang-(1–9) and alamandine48 however these compounds rapidly degrade and thus could not be measured in our samples. Also, we did not administer rhACE2 to healthy controls to measure the effect on oxidant stress of inflammatory compounds, however, literature supports comparing an individuals own cytokine panel before and after intervention, rather than comparing groups given variability in these measurements49, 50.
In conclusion, our data show evidence of reduced ACE2 activity in human PAH accompanied by reduced SOD2 activity, increased cytokine expression and increased oxidant stress. Infusion of a single dose of recombinant human ACE2 was well-tolerated and may have potential hemodynamic benefit. GSK2586881 administration was associated with increased SOD2 levels, reduction in markers of inflammation and reduced plasma oxidant stress. Further study of GSK2586881 as a therapeutic in PAH is warranted.
Supplementary Material
Take Home Message:
The AngII/Ang-(1–7)/Mas1 axis is likely involved in the pathophysiology of human pulmonary arterial hypertension.
Acknowledgements:
The authors wish to thank Aili L. Lazaar, MD and Kelly Hardes for their assistance in planning the clinical studies, data analysis, and providing review of the manuscript.
Funding Sources:
This work was supported by NHLBI P01-HL-108800. The project was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. GlaxoSmithKline provided investigational compound and in-kind support in the form of RAS peptide assays.
Funding: NHLBI P01-HL-108800, UL1TR000445, GlaxoSmithKline provided investigational compound and in-kind support in the form of RAS peptide assays
References:
- 1.de Man FS, Tu L, Handoko ML, Rain S, Ruiter G, Francois C, Schalij I, Dorfmuller P, Simonneau G, Fadel E, Perros F, Boonstra A, Postmus PE, van der Velden J, Vonk-Noordegraaf A, Humbert M, Eddahibi S and Guignabert C. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrell NW, Danilov SM, Satyan KB, Morris KG and Stenmark KR. Right ventricular angiotensin converting enzyme activity and expression is increased during hypoxic pulmonary hypertension. Cardiovasc Res. 1997;34:393–403. [DOI] [PubMed] [Google Scholar]
- 3.Morrell NW, Atochina EN, Morris KG, Danilov SM and Stenmark KR. Angiotensin converting enzyme expression is increased in small pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. J Clin Invest. 1995;96:1823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrell NW, Morris KG and Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269:H1186–94. [DOI] [PubMed] [Google Scholar]
- 5.Alpert MA, Pressly TA, Mukerji V, Lambert CR and Mukerji B. Short- and long-term hemodynamic effects of captopril in patients with pulmonary hypertension and selected connective tissue disease. Chest. 1992;102:1407–12. [DOI] [PubMed] [Google Scholar]
- 6.Ikram H, Maslowski AH, Nicholls MG, Espiner EA and Hull FT. Haemodynamic and hormonal effects of captopril in primary pulmonary hypertension. British heart journal. 1982;48:541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W and Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60–72. [DOI] [PubMed] [Google Scholar]
- 8.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M and Hoeper M. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev Esp Cardiol (Engl Ed). 2016;69:177. [DOI] [PubMed] [Google Scholar]
- 9.Lambert DW, Hooper NM and Turner AJ. Angiotensin-converting enzyme 2 and new insights into the renin-angiotensin system. Biochem Pharmacol. 2008;75:781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tallant EA, Lu X, Weiss RB, Chappell MC and Ferrario CM. Bovine aortic endothelial cells contain an angiotensin-(1–7) receptor. Hypertension. 1997;29:388–93. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB and Diz DI. Counterregulatory actions of angiotensin-(1–7). Hypertension. 1997;30:535–41. [DOI] [PubMed] [Google Scholar]
- 12.Brosnihan KB, Li P and Ferrario CM. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–8. [DOI] [PubMed] [Google Scholar]
- 13.Nakamoto H, Ferrario CM, Fuller SB, Robaczewski DL, Winicov E and Dean RH. Angiotensin-(1–7) and nitric oxide interaction in renovascular hypertension. Hypertension. 1995;25:796–802. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Liu J, Luo JY, Tian XY, Cheang WS, Xu J, Lau CW, Wang L, Wong WT, Wong CM, Lan HY, Yao X, Raizada MK and Huang Y. Upregulation of Angiotensin (1–7)-Mediated Signaling Preserves Endothelial Function Through Reducing Oxidative Stress in Diabetes. Antioxid Redox Signal. 2015;23:880–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M and Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins IM, Morrow JD and Christman BW. Oxidant stress but not thromboxane decreases with epoprostenol therapy. Free Radic Biol Med. 2005;38:568–74. [DOI] [PubMed] [Google Scholar]
- 17.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM and Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. The New England journal of medicine. 1992;327:70–5. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, Harral JW, Talati M, Lane KB, Fagan KA and West J. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JA, West J, Maynard KB and Hemnes AR. ACE2 improves right ventricular function in a pressure overload model. PLoS One. 2011;6:e20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenoy V, Gjymishka A, Jarajapu YP, Qi Y, Afzal A, Rigatto K, Ferreira AJ, Fraga-Silva RA, Kearns P, Douglas JY, Agarwal D, Mubarak KK, Bradford C, Kennedy WR, Jun JY, Rathinasabapathy A, Bruce E, Gupta D, Cardounel AJ, Mocco J, Patel JM, Francis J, Grant MB, Katovich MJ and Raizada MK. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013;187:648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ and Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Diez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK and Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med. 2010;182:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breitling S, Krauszman A, Parihar R, Walther T, Friedberg MK and Kuebler WM. Dose-dependent, therapeutic potential of angiotensin-(1–7) for the treatment of pulmonary arterial hypertension. Pulm Circ. 2015;5:649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Xiao L, Li F, Zhang H, Li Q, Liu H, Fu S, Li C, Zhang X, Wang J, Staunstrup NH, Li Y and Yang H. Generation of outbred Ace2 knockout mice by RNA transfection of TALENs displaying colitis reminiscent pathophysiology and inflammation. Transgenic Res. 2015;24:433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, Bruggisser M, Penninger J and Krahenbuhl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52:783–92. [DOI] [PubMed] [Google Scholar]
- 26.Fike CD, Aschner JL, Zhang Y and Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1244–54. [DOI] [PubMed] [Google Scholar]
- 27.West JD, Austin ED, Gaskill C, Marriott S, Baskir R, Bilousova G, Jean JC, Hemnes AR, Menon S, Bloodworth NC, Fessel JP, Kropski JA, Irwin D, Ware LB, Wheeler L, Hong CC, Meyrick B, Loyd JE, Bowman AB, Ess KC, Klemm DJ, Young PP, Merryman WD, Kotton D and Majka SM. Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. American journal of physiology Cell physiology. 2014;307:C415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin ED, Menon S, Hemnes AR, Robinson LR, Talati M, Fox KL, Cogan JD, Hamid R, Hedges LK, Robbins I, Lane K, Newman JH, Loyd JE and West J. Idiopathic and heritable PAH perturb common molecular pathways, correlated with increased MSX1 expression. Pulm Circ. 2011;1:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM and Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41. [DOI] [PubMed] [Google Scholar]
- 30.Barnes PJ and Liu SF. Regulation of pulmonary vascular tone. Pharmacological reviews. 1995;47:87–131. [PubMed] [Google Scholar]
- 31.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, Dartevelle P, Simonneau G, Adnot S and Eddahibi S. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176:1041–7. [DOI] [PubMed] [Google Scholar]
- 32.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N and Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG and Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. American journal of physiology Heart and circulatory physiology. 2008;294:H570–8. [DOI] [PubMed] [Google Scholar]
- 34.Nozik-Grayck E, Woods C, Taylor JM, Benninger RK, Johnson RD, Villegas LR, Stenmark KR, Harrison DG, Majka SM, Irwin D and Farrow KN. Selective depletion of vascular EC-SOD augments chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;307:L868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J and Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–7. [DOI] [PubMed] [Google Scholar]
- 36.Roberts LJ 2nd, Fessel JP and Davies SS. The biochemistry of the isoprostane, neuroprostane, and isofuran Pathways of lipid peroxidation. Brain Pathol. 2005;15:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price LC, Wort SJ, Perros F, Dorfmuller P, Huertas A, Montani D, Cohen-Kaminsky S and Humbert M. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–21. [DOI] [PubMed] [Google Scholar]
- 38.Price LC, Montani D, Tcherakian C, Dorfmuller P, Souza R, Gambaryan N, Chaumais MC, Shao DM, Simonneau G, Howard LS, Adcock IM, Wort SJ, Humbert M and Perros F. Dexamethasone reverses monocrotaline-induced pulmonary arterial hypertension in rats. Eur Respir J. 2011;37:813–22. [DOI] [PubMed] [Google Scholar]
- 39.Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME and Tikellis C. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res. 2010;107:888–97. [DOI] [PubMed] [Google Scholar]
- 40.da Silveira KD, Coelho FM, Vieira AT, Sachs D, Barroso LC, Costa VV, Bretas TL, Bader M, de Sousa LP, da Silva TA, dos Santos RA, Simoes e Silva AC and Teixeira MM. Anti-inflammatory effects of the activation of the angiotensin-(1–7) receptor, MAS, in experimental models of arthritis. J Immunol. 2010;185:5569–76. [DOI] [PubMed] [Google Scholar]
- 41.Jankov RP, Kantores C, Pan J and Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2008;294:L233–45. [DOI] [PubMed] [Google Scholar]
- 42.Toporsian M, Jerkic M, Zhou YQ, Kabir MG, Yu LX, McIntyre BA, Davis A, Wang YJ, Stewart DJ, Belik J, Husain M, Henkelman M and Letarte M. Spontaneous adult-onset pulmonary arterial hypertension attributable to increased endothelial oxidative stress in a murine model of hereditary hemorrhagic telangiectasia. Arterioscler Thromb Vasc Biol. 2010;30:509–17. [DOI] [PubMed] [Google Scholar]
- 43.Wood KC, Hsu LL and Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506–28. [DOI] [PubMed] [Google Scholar]
- 44.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J and Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giaid A and Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. The New England journal of medicine. 1995;333:214–21. [DOI] [PubMed] [Google Scholar]
- 46.Champion HC, Bivalacqua TJ, D’Souza FM, Ortiz LA, Jeter JR, Toyoda K, Heistad DD, Hyman AL and Kadowitz PJ. Gene transfer of endothelial nitric oxide synthase to the lung of the mouse in vivo. Effect on agonist-induced and flow-mediated vascular responses. Circ Res. 1999;84:1422–32. [DOI] [PubMed] [Google Scholar]
- 47.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC and Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–9. [DOI] [PubMed] [Google Scholar]
- 48.Jankowski V, Vanholder R, van der Giet M, Tolle M, Karadogan S, Gobom J, Furkert J, Oksche A, Krause E, Tran TN, Tepel M, Schuchardt M, Schluter H, Wiedon A, Beyermann M, Bader M, Todiras M, Zidek W and Jankowski J. Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arterioscler Thromb Vasc Biol. 2007;27:297–302. [DOI] [PubMed] [Google Scholar]
- 49.Hosnijeh FS, Krop EJ, Portengen L, Rabkin CS, Linseisen J, Vineis P and Vermeulen R. Stability and reproducibility of simultaneously detected plasma and serum cytokine levels in asymptomatic subjects. Biomarkers. 2010;15:140–8. [DOI] [PubMed] [Google Scholar]
- 50.Biancotto A, Wank A, Perl S, Cook W, Olnes MJ, Dagur PK, Fuchs JC, Langweiler M, Wang E and McCoy JP. Baseline levels and temporal stability of 27 multiplexed serum cytokine concentrations in healthy subjects. PLoS One. 2013;8:e76091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.