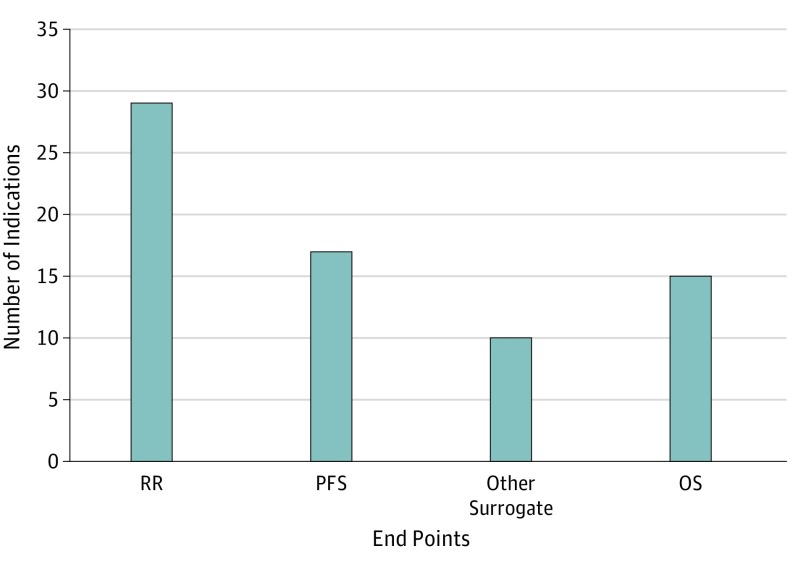
Figure 1. Overall Survival and Surrogate End Points Used as the Basis of Approval for 69 Initial Indications of 63 Novel Oncology Drugs Approved by the FDA Between 2011 and 2017a,b,c.
FDA indicates the US Food and Drug Administration; OS, overall survival; PFS, progression-free survival; RR, response rate. Other surrogates include event-free survival, invasive disease-free survival, major cytogenetic response, major hematologic response, complete hematologic response, overall hematologic response, complete remission/complete remission with partial hematological recovery, complete response/complete response with partial hematologic recovery, and complete remission/incomplete remission.
aInstead of approval based on OS or a surrogate, ruxolitinib and asparaginase Erwinia chrysanthemi were approved on the basis of spleen volume reduction and asparaginase activity level, respectively.
bResponse rate and PFS were coprimary end points of the pivotal trial for elotuzumab; both end points were reached.
cResponse rate was the primary end point of the abemaciclib monotherapy trial while PFS was the primary end point of the abemaciclib-fulvestrant combination trial.