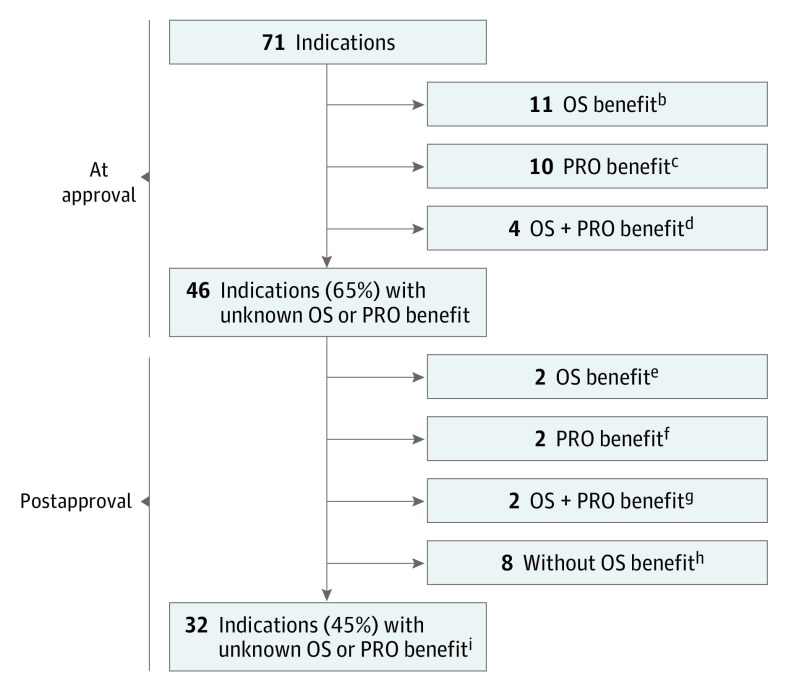
Figure 2. OS and PRO Benefits for 71 Initial Indications of 65 Novel Oncology Drugs Approved by the FDA Between 2011 and 2017a.
FDA indicates the US Food and Drug Administration; OS, overall survival; PRO, patient-reported outcome.
aA drug was considered to have demonstrated an OS benefit if OS was a prespecified primary or secondary end point of a pivotal trial (or postapproval study) and a statistically significant difference was observed between the drug and comparator arms. A drug was considered to have demonstrated an improvement in a PRO if a statistically significant difference was reported between the drug and comparator arms for a global score, a subscale, or a specific item from a validated PRO instrument evaluated in the trial(s).
bMidostaurin (indication: acute myeloid leukemia), olaratumab, necitumumab, cobimetinib, trifluridine/tipiracil, ramucirumab, regorafenib, ziv-aflibercept, pertuzumab, vemurafenib, and ipilimumab.
cInotuzumab ozogamicin, pembrolizumab, ceritinib, pomalidomide, dabrafenib, trametinib, afatinib, crizotinib, ruxolitinib, and vandetanib.
dAdo-trastuzumab emtansine, radium 223, enzalutamide, and abiraterone acetate.
eIxazomib and obinutuzumab (OS benefit was demonstrated for a total of 6 indications postapproval; 4 [pembrolizumab, pomalidomide, trametinib, and ruxolitinib] had previously demonstrated a PRO benefit).
fOsimertinib and ibrutinib.
gBlinatumomab and idelalisib (indication: chronic lymphocytic leukemia).
hPanobinostat, trabectedin, lenvatinib, nivolumab, olaparib, cabozantinib, atezolizumab, and axitinib (no OS benefit was shown for a total of 13 indications; 5 [afatinib, crizotinib, vandetanib, ibrutinib, and inotuzumab ozogamicin] had previously demonstrated a PRO benefit). For 5 indications (afatinib, axitinib, inotuzumab ozogamicin, lenvatinib, and trabectedin), the lack of OS benefit was known at the time of initial approval.
iDurvalumab, ribociclib, brigatinib, midostaurin (indication: aggressive systemic mastocytosis), enasidenib, acalabrutinib, copanlisib, avelumab (2 indications), neratinib, abemaciclib, niraparib, rucaparib, venetoclax, daratumumab, alectinib, sonidegib, dinutuximab, belinostat, bosutinib, vismodegib, palbociclib, elotuzumab, ponatinib (2 indications), carfilzomib, omacetaxine, asparaginase Erwinia chrysanthemi, brentuximab (2 indications), and idelalisib (indications: follicular B-cell non–Hodgkin lymphoma and small lymphocytic lymphoma).