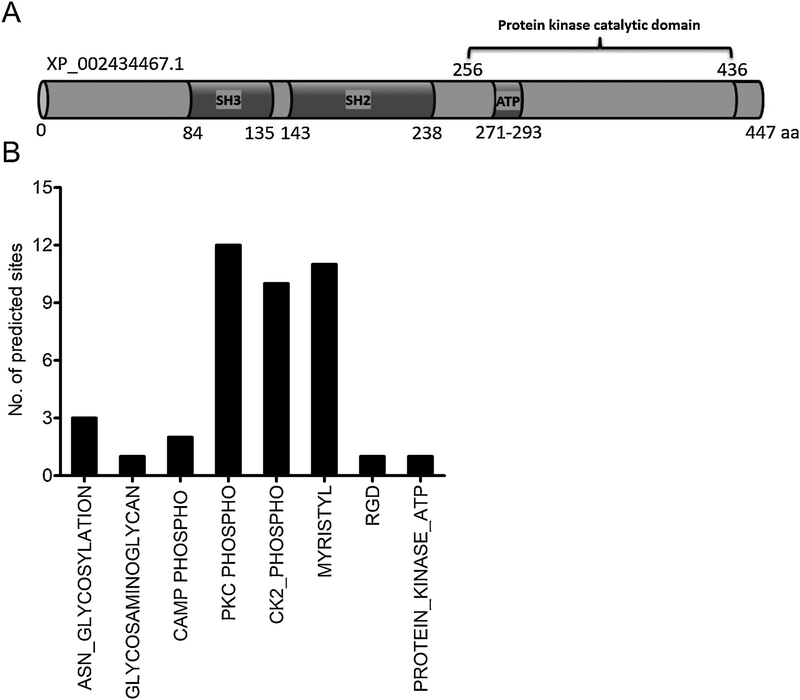
Figure 2: Domain analysis and prediction of modification sites in I. scapularis Src kinase.
A) Domain analysis of I. scapularis Src kinase primary amino acid sequence at PROSITE (ExPASy) and NCBI protein database is shown. Structurally, I. scapularis Src consist of the SH3 domain, a SH3-SH2 connector, a SH2 domain, a SH2-kinase linker, a kinase domain, Protein kinases ATP-binding region signature and a C-terminal tail regulatory region. The phosphorylation of tyrosine site (Tyr416 in the catalytic domain and C-terminal region) conserved in the I. scapularis Src. B) Bioinformatic analysis showing I. scapularis Src kinase modification sites and relevant amino acids. I. scapularis Src kinase, contain three Asparagine (ASN)-glycosylation sites, one glycosaminoglycan site, two cAMP phosphorylation sites, twelve PKC phosphorylation sites, ten CK2 phosphorylation sites, eleven myristoylation sites, one RGD site and one protein kinase ATP binding site.