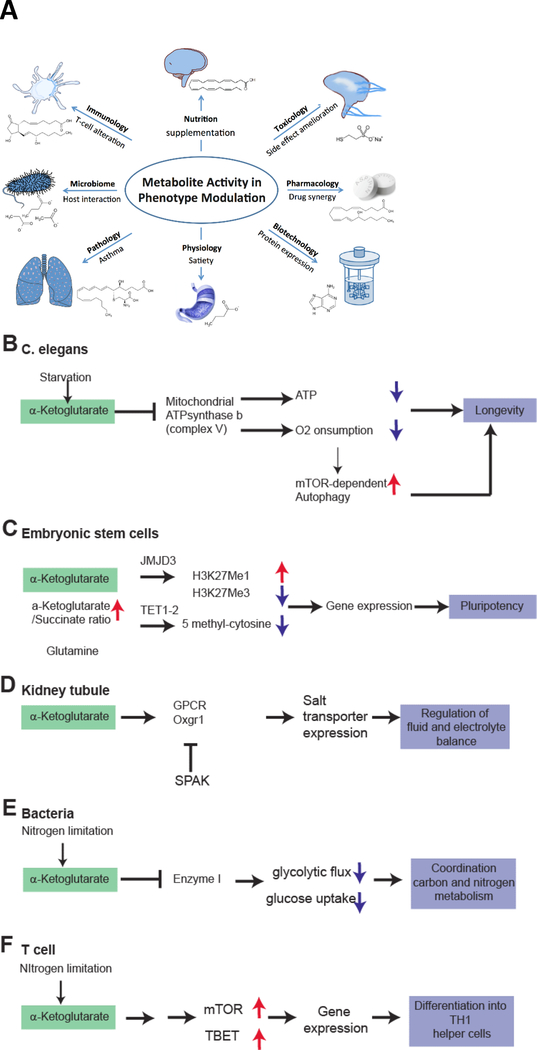
Figure 5.
A. Metabolite Activity for Phenotype Modulation. Metabolomics has already made a significant impact in a wide variety of scientific areas through discovery of active, mainly endogenous metabolites that can regulate different biological processes and thus modulate the phenotype in health and disease. B-F. Metabolic activity of α-ketoglutarate (AKG) in a variety of prokaryote to eukaryote systems. AKG accumulates in exercise, but can also be supplemented. Direct supplementation has a wide effect on various systems via different mechanisms. Common primary AKG sensors may be ATPase b subunit (as revealed by DARTs proteomics) or PII (in plants/prokaryotes). B. In C. elegans, AKG addition leads to binding of the ATPase b subunit to inhibit ATP production, oxygen consumption, and thereby stimulating mTOR dependent autophagy. All of these mechanisms result in an extended life span of the worm. C. In embryonic stem cells, an increased AKG/Succinate ratio drives activation of JMJD3 and TET1/2 to perform epigenetic changes. These include a reduced trimethylation at H3K27Me3 and also a reduced methylation of DNA (5-methylcytosine reduced). This enables increased gene expression required for pluripotency. D. In specialized mammalian organs such as the kidney, AKG is being secreted to control organ function in a paracellular manner. Urinary AKG derived from metabolic stress results in activation of Oxgr1 and subsequent activation of salt transporters to regulate electrolyte balance and, presumably, hypertension. E. In bacteria, intracellular AKG induced by nitrogen limitation inhibits Enzyme 1 to decrease glycolytic flux and to couple nitrogen consumption to glucose consumption. F. In T-cells, AKG induces activity of mTOR and the T-cell specific transcription factor TBET via unknown mechanism to promote differentiation to TH1 cells over TH2 cells.