FIGURE 5.
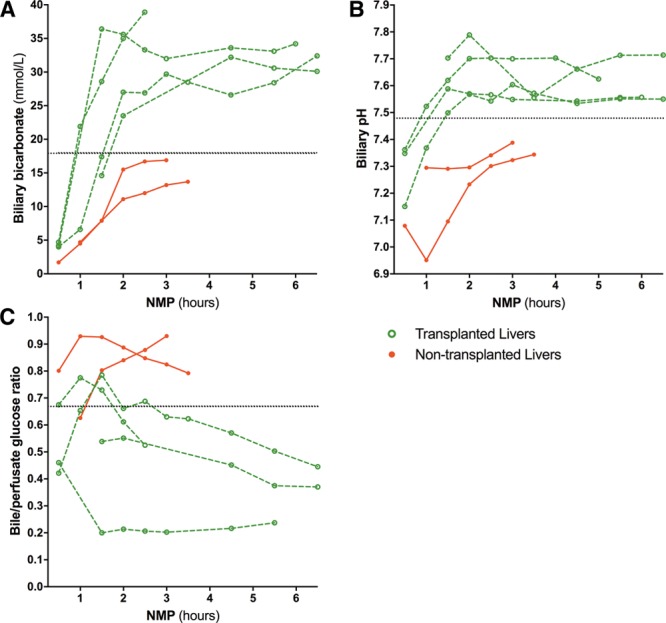
Clinical validation of biliary biomarkers during ex situ NMP of transplanted and nontransplanted livers. Six livers that were declined for transplantation nationwide underwent end-ischemic NMP to assess their viability for transplantation in a clinical trial. According to protocol, viability assessment occurred within 2.5 hours NMP. Four livers fulfilled the hepatobiliary criteria and were transplanted. There was no clinical evidence of posttransplant cholangiopathy at a median of 8.3 months (IQR, 7.6-10.1) follow-up. Dotted lines indicate biliary biomarker cutoff values established in the preclinical study. IQR, interquartile range; NMP, normothermic machine perfusion.
