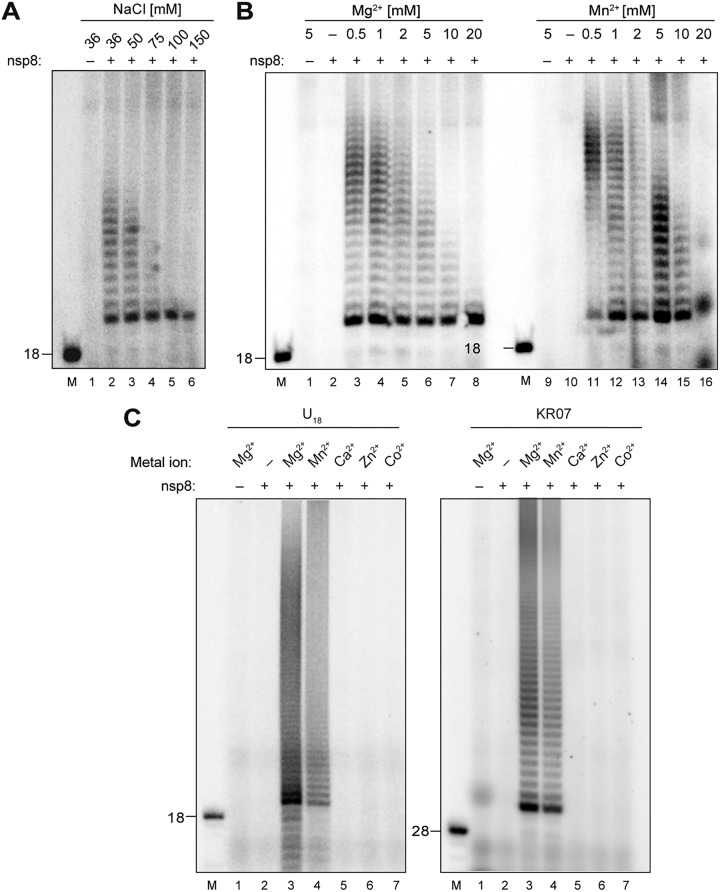
FIG 2.
RNA 3′-terminal nucleotidyl transferase (TNTase) activity of HCoV-229E nsp8. The effects of increasing salt concentrations and the presence of metal ions on TNTase activity were determined. (A) nsp8 activity assays were performed in reaction buffer supplemented with 2 μM nsp8, 1 μM U18 (substrate RNA), 100 μM ATP, 0.17 μM [α-32P]ATP, 1 mM MgCl2, and various concentrations of NaCl (36 to 150 mM). (B) Activity assays were performed in reaction buffer supplemented with 2 μM nsp8, 50 mM NaCl, 100 μM ATP, 0.17 μM [α-32P]ATP, 1 μM U18 (substrate RNA), and the indicated concentrations of MgCl2 and MnCl2 (0 to 20 mM). (C) Activity assays were performed in reaction buffer supplemented with 2 μM nsp8, 50 mM NaCl, 100 μM ATP, 0.17 μM [α-32P]ATP, and 1 μM U18 (left) or KR07 RNA (right), and the indicated divalent metal ions (each at 1 mM). 5′-32P-labeled RNAs of U18 and KR07 were used as 18-nt and 28-nt markers, respectively, as indicated to the left. The reaction mixtures were incubated at 30°C for 60 min. Products were resolved in a TBE-buffered 12% polyacrylamide–7 M urea gel and visualized by phosphorimaging. Lanes M, 5′-32P-labeled RNAs (U18, KR07) were used as markers with sizes (in nucleotides) indicated to the left.